ASTM F451-99ae1
(Specification)Standard Specification for Acrylic Bone Cement
Standard Specification for Acrylic Bone Cement
SCOPE
1.1 This specification covers self-curing resins used primarily for the fixation of internal orthopedic prostheses. The mixture may be used in either the predough or dough stage in accordance with the manufacturer's recommendations.
1.2 Units of premeasured powder and liquid are supplied in a form suitable for mixing. The mixture then sets in place.
1.3 While a variety of copolymers and comonomers may be incorporated, the composition of the set cement shall contain poly(methacrylic acid esters) as its main ingredient.
1.4 This specification covers compositional, physical performance, and biocompatibility as well as packaging requirements. The biocompatibility of acrylic bone cement as it has been traditionally formulated and used has been reported in the literature (1, 2).
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
e1
Designation: F 451 – 99a
Standard Specification for
1
Acrylic Bone Cement
This standard is issued under the fixed designation F 451; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1
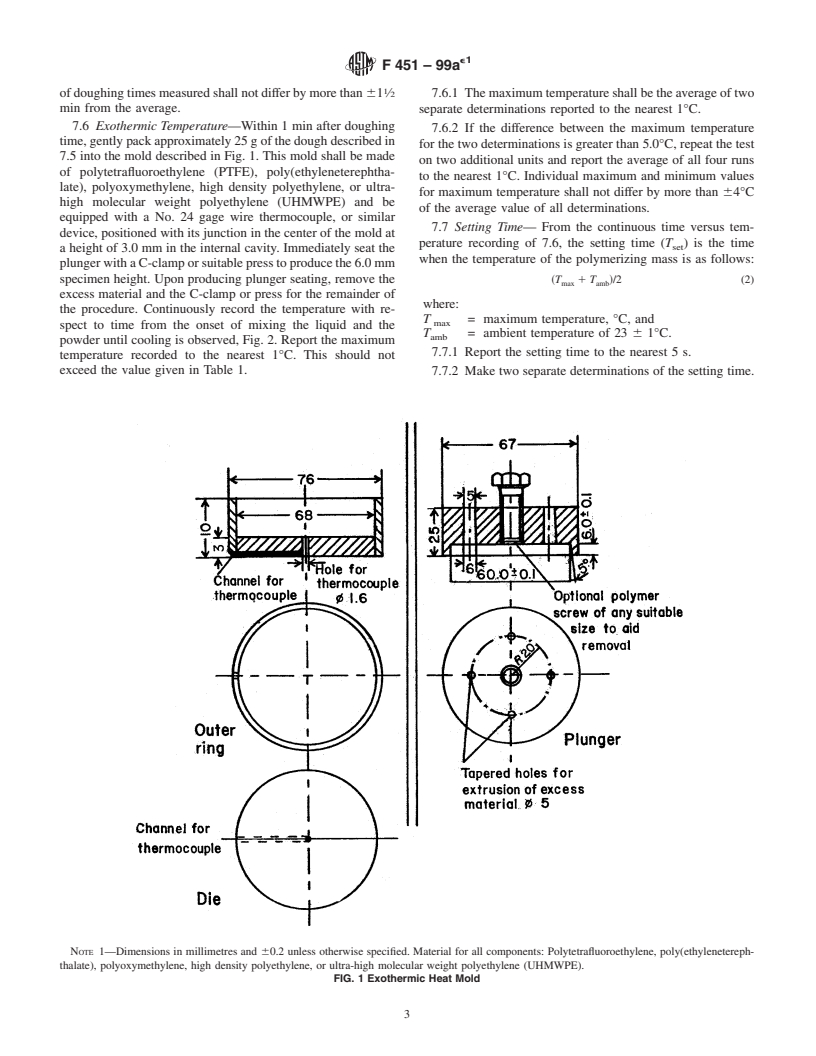
e NOTE—Figure 1 was editorially corrected in June 2003.
1. Scope F 748 Practice for Selecting Generic Biological Test Meth-
6
ods for Materials and Devices
1.1 This specification covers self-curing resins used prima-
F 749 Practice for Evaluating Material Extracts by Intracu-
rily for the fixation of internal orthopedic prostheses. The
6
taneous Injection in the Rabbit
mixture may be used in either the predough or dough stage in
F 756 Practice for Assessment of Hemolytic Properties of
accordance with the manufacturer’s recommendations.
6
Materials
1.2 Units of premeasured powder and liquid are supplied in
F 763 Practice for Short-Term Screening of Implant Mate-
a form suitable for mixing. The mixture then sets in place.
6
rials
1.3 While a variety of copolymers and comonomers may be
F 813 PracticeforDirectContactCellCultureEvaluationof
incorporated, the composition of the set cement shall contain
6
Materials for Medical Devices
poly(methacrylic acid esters) as its main ingredient.
F 895 Practice for Agar Diffusion Cell Culture Screening
1.4 This specification covers compositional, physical per-
6
for Cytotoxicity
formance, and biocompatibility as well as packaging require-
F 981 Practice for Assessment of Compatibility of Bioma-
ments. The biocompatibility of acrylic bone cement as it has
terials (Nonporous) for Surgical Implants with Respect to
been traditionally formulated and used has been reported in the
6
2
Effect of Materials on Muscle and Bone
literature (1, 2).
2.2 ANSI/ADA Standard:
1.5 This standard does not purport to address all of the
7
No. 15 Specification for Acrylic Resin Teeth
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
3. Terminology
priate safety and health practices and determine the applica-
3.1 Definitions of Terms Specific to This Standard:
bility of regulatory limitations prior to use.
3.1.1 doughing time—the time after commencement of
2. Referenced Documents mixing at which the mixture ceases to adhere to a standard
probe (see 7.5).
2.1 ASTM Standards:
3.1.2 exothermic or maximum temperature—the maximum
D 695 Test Method for Compressive Properties of Rigid
3
temperature of the mixture due to self-curing in a standard
Plastics
mold (see 7.6).
D 3835 Test Method for Determination of Properties of
4
3.1.3 extrusion—the rate of flow of the material through a
Polymeric Materials by Means of a Capillary Rheometer
standard orifice under load (see 7.8.1).
E 29 Practice for Using Significant Digits in Test Data to
5
3.1.4 intrusion—the distance of flow of the mixture into a
Determine Conformance With Specifications
standard mold under load (see 7.8.2).
E 141 Practice for Acceptance of Evidence Based on the
5
3.1.5 setting time—the time after commencement of mixing
Results of Probability Sampling
6 at which the temperature of the curing mass equals the average
F 619 Practice for Extraction of Medical Plastics
of the maximum and ambient temperatures (see 7.7).
3.1.6 unit—one package or vial of premeasured powder
1 component and one package or vial of premeasured liquid
This specification is under the jurisdiction of ASTM Committee F04 on
component.
Medical and Surgical Materials and Devices and is the direct responsibility of
Subcommittee F04.11 on Polymeric Materials.
Current edition approved May 10, 1999. Published July 1999. Originally
4. Physical Requirements
published as F 451 – 76. Last previous edition F 451 – 95.
2 4.1 Liquid:
The boldface numbers in parentheses refer to the list of references at the end of
this standard.
3
Annual Book of ASTM Standards, Vol 08.01.
4
Annual Book of ASTM Standards, Vol 08.02.
5 7
Annual Book of ASTM Standards, Vol 14.02. Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
6
Annual Book of ASTM Standards, Vol 13.01. 4th Floor, New York, NY 10036.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
e1
F 451 – 99a
TABLE 2 Requirements for Cured Polymer After Setting
4.1.1 Appearance—The liquid shall be free of extraneous
particulate matter or obvious visual contaminants in its con- Property Requirement
tainer.
Compressive Strength, min., MPa 70
4.1.2 Stability—Afterbeingheatedfor48hat60 62°C,the
viscosity of the liquid shall not increase by more than 1
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.