ASTM F2118-14(2020)
(Test Method)Standard Test Method for Constant Amplitude of Force Controlled Fatigue Testing of Acrylic Bone Cement Materials
Standard Test Method for Constant Amplitude of Force Controlled Fatigue Testing of Acrylic Bone Cement Materials
SIGNIFICANCE AND USE
5.1 This test method describes a uniaxial, constant amplitude, fully reversed fatigue test to characterize the fatigue performance of a uniform cylindrical waisted specimen manufactured from acrylic bone cement.
5.2 This test method considers two approaches to evaluating the fatigue performance of bone cement:
5.2.1 Testing is conducted at three stress levels to characterize the general fatigue behavior of a cement over a range of stresses. The stress level and resultant cycles to failure of the specimens can be plotted on an S-N diagram.
5.2.2 Another approach is to determine the fatigue life of a particular cement. The fatigue life for orthopaedic bone cement is to be determined up to 5 million (5 × 106) cycles.
5.3 This test method does not define or suggest required levels of performance of bone cement. This fatigue test method is not intended to represent the clinical use of orthopaedic bone cement, but rather to characterize the material using standard and well-established methods. The user is cautioned to consider the appropriateness of this test method in view of the material being tested and its potential application.
5.4 It is widely reported that multiple clinical factors affect the fatigue performance of orthopaedic bone cement; however, the actual mechanisms involves multiple factors. Clinical factors which may affect the performance of bone cement include: temperature and humidity, mixing method, time of application, surgical technique, bone preparation, implant design, anatomical site, and patient factors, among others. This test method does not specifically address all of these clinical factors. The test method can be used to compare different acrylic bone cement formulations and products and different mixing methods and environments (that is, mixing temperature, vacuum, centrifugation, and so forth).
SCOPE
1.1 This test method describes test procedures for evaluating the constant amplitude, uniaxial, tension-compression uniform fatigue performance of acrylic bone cement materials.
1.2 This test method is relevant to orthopedic bone cements based on acrylic resins, as specified in Specification F451 and ISO 16402. The procedures in this test method may or may not apply to other surgical cement materials.
1.3 It is not the intention of this test method to define levels of performance of these materials. It is not the intention of this test method to directly simulate the clinical use of these materials, but rather to allow for comparison between acrylic bone cements to evaluate fatigue behavior under specified conditions.
1.4 A rationale is given in Appendix X2.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2118 − 14 (Reapproved 2020)
Standard Test Method for
Constant Amplitude of Force Controlled Fatigue Testing of
Acrylic Bone Cement Materials
This standard is issued under the fixed designation F2118; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This test method describes test procedures for evaluat-
D412TestMethodsforVulcanizedRubberandThermoplas-
ing the constant amplitude, uniaxial, tension-compression uni-
tic Elastomers—Tension
form fatigue performance of acrylic bone cement materials.
D792Test Methods for Density and Specific Gravity (Rela-
1.2 This test method is relevant to orthopedic bone cements
tive Density) of Plastics by Displacement
based on acrylic resins, as specified in Specification F451 and
D1084Test Methods for Viscosity of Adhesives
ISO16402.Theproceduresinthistestmethodmayormaynot
D2090Test Method for Clarity and Cleanness of Paint and
apply to other surgical cement materials.
Ink Liquids (Withdrawn 2007)
D2196Test Methods for Rheological Properties of Non-
1.3 It is not the intention of this test method to define levels
Newtonian Materials by Rotational Viscometer
of performance of these materials. It is not the intention of this
D2240Test Method for Rubber Property—Durometer Hard-
test method to directly simulate the clinical use of these
ness
materials, but rather to allow for comparison between acrylic
E466Practice for Conducting Force Controlled Constant
bone cements to evaluate fatigue behavior under specified
Amplitude Axial Fatigue Tests of Metallic Materials
conditions.
E467Practice for Verification of Constant Amplitude Dy-
1.4 A rationale is given in Appendix X2. namic Forces in an Axial Fatigue Testing System
E1823TerminologyRelatingtoFatigueandFractureTesting
1.5 The values stated in SI units are to be regarded as
F451Specification for Acrylic Bone Cement
standard. No other units of measurement are included in this
2.2 ISO Standard:
standard.
ISO 16402Flexural Fatigue Testing of Acrylic Resin Ce-
1.6 This standard does not purport to address all of the
ments Used in Orthopedics
safety concerns, if any, associated with its use. It is the
3. Terminology
responsibility of the user of this standard to establish appro-
priate safety, health, and environmental practices and deter-
3.1 Unless otherwise given, the definitions for fatigue ter-
mine the applicability of regulatory limitations prior to use.
minology given in Terminology E1823 will be used.
1.7 This international standard was developed in accor-
3.2 Definitions:
dance with internationally recognized principles on standard-
3.2.1 mean fatigue life at N cycles—the average number of
ization established in the Decision on Principles for the
cycles to failure at the specified load level. For the purposes of
Development of International Standards, Guides and Recom-
thistestmethod,thefatiguelifewillbedeterminedat5million
mendations issued by the World Trade Organization Technical
load cycles. A rationale for this is provided in X2.4.
Barriers to Trade (TBT) Committee.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
ThistestmethodisunderthejurisdictionofASTMCommitteeF04onMedical Standards volume information, refer to the standard’s Document Summary page on
andSurgicalMaterialsandDevicesandisthedirectresponsibilityofSubcommittee the ASTM website.
F04.15 on Material Test Methods. The last approved version of this historical standard is referenced on
Current edition approved Sept. 1, 2020. Published September 2020. Originally www.astm.org.
approved in 2001. Last previous edition approved in 2014 as F2118–14. Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
DOI:10.1520/F2118-14R20. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F2118 − 14 (2020)
3.2.2 median fatigue life at a given stress level—thenumber mixing methods and environments (that is, mixing
of cycles to failure at which 50% of the tested samples failed temperature, vacuum, centrifugation, and so forth).
at the specified stress level.
6. Apparatus
3.2.3 runout—a predetermined number of cycles at which
the testing on a particular specimen will be stopped, and no
6.1 Uniaxial Load Frame—A testing machine capable of
further testing on that specimen will be performed. For the
applying cyclic sinusoidal tensile and compressive loads.
purposes of this test method, the runout will be 5 million load
6.1.1 Thecrossheadsoftheloadframeshallbealignedsuch
cycles.
that the alignment meets the requirements of section 8.2 of
Practice E466. The alignment should be checked at both the
3.2.4 specimenfailure—theconditionatwhichthespecimen
maximumtensileandminimumcompressiveloadtobeapplied
completelybreaksorisdamagedtosuchanextentthattheload
during the course of a test program.
frame is no longer able to apply the intended stress within the
required limits.
6.2 Cycle Counter—Adevice capable of counting the num-
ber of loading cycles applied to a specimen during the course
3.2.5 stress level—the value of stress at which a series of
duplicate tests are performed. For the purposes of this test of a fatigue test.
method, the stress level is reported as the maximum stress
6.3 Load Cell—A load cell capable of measuring dynamic
applied to the specimen.
tensile and compressive loads in accordance with Practice
E467.
4. Summary of Test Method
6.4 Limit—A device capable of detecting when a test pa-
4.1 Uniformcylindricalreducedgagesectiontestspecimens
rameter (for example, load magnitude, actuator displacement,
are manufactured from acrylic bone cement and mounted in a
directcurrent(DC)error,andsoforth)reachesalimitingvalue,
uniaxial fatigue frame. The specimen is subjected to fully
at which time the test is stopped and the current cycle count
reversed tensile and compressive loading in a sinusoidal cyclic
recorded.
manner at a specified frequency in phosphate buffered saline
(PBS).Thefatigueloadingiscontinueduntilthespecimenfails 6.5 Environmental Chamber—A chamber designed to im-
merse the fatigue specimen completely in a solution. The
orapredeterminednumberofcycles(run-outlimit)isreached.
chamber should have provisions for maintaining a constant
5. Significance and Use
temperature to an accuracy of 62°C.
5.1 This test method describes a uniaxial, constant
amplitude,fullyreversedfatiguetesttocharacterizethefatigue 7. Test Specimen
performance of a uniform cylindrical waisted specimen manu-
7.1 Test specimens shall be fabricated from cement that is
factured from acrylic bone cement.
representative of the final product with regard to materials,
5.2 Thistestmethodconsiderstwoapproachestoevaluating manufacturing processes, sterilization, and packaging. Certain
sterilization methods (for example, gamma sterilization of the
the fatigue performance of bone cement:
5.2.1 Testing is conducted at three stress levels to charac- powder) have been shown to have an effect on fatigue
performance. Any deviations of the test cement from the
terize the general fatigue behavior of a cement over a range of
stresses. The stress level and resultant cycles to failure of the clinically used product must be reported.
specimens can be plotted on an S-N diagram.
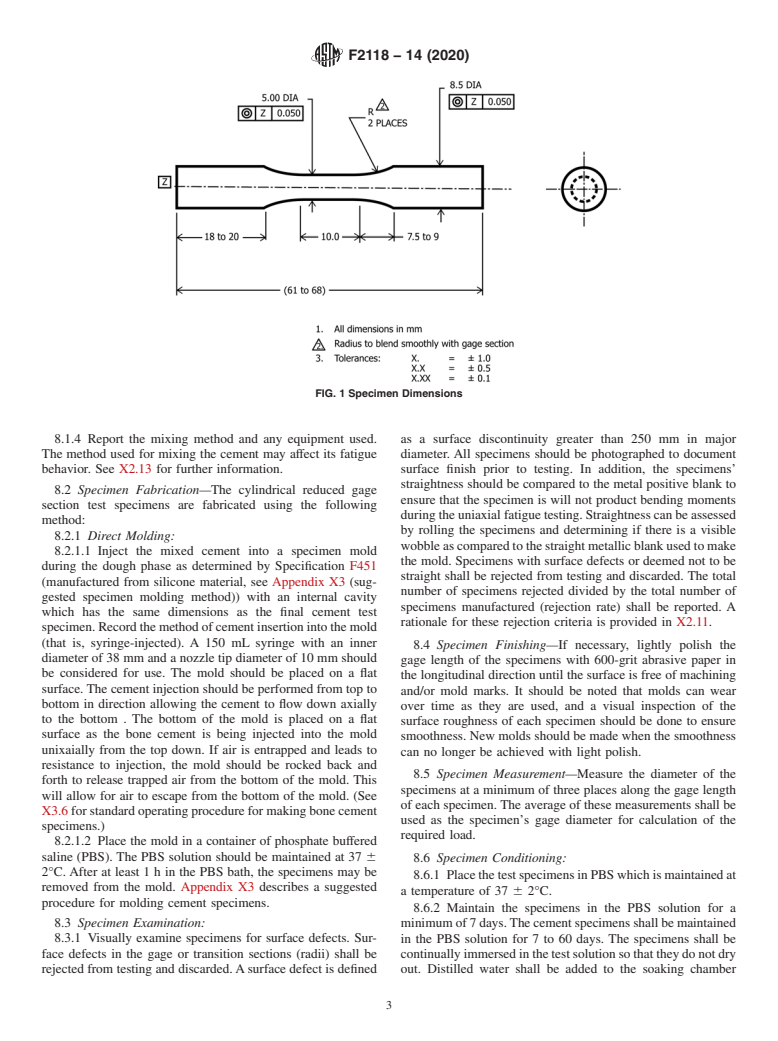
7.2 Cylindrical reduced gage section test specimens with a
5.2.2 Another approach is to determine the fatigue life of a
straight 5-mm diameter by 10-mm-long gage section shall be
particularcement.Thefatiguelifefororthopaedicbonecement
used. The diameter of the specimen ends shall be substantially
is to be determined up to 5 million (5 × 10 ) cycles.
greater than the gage diameter to ensure that fracture occurs in
the gage section.Asmooth surface of the test specimen in the
5.3 This test method does not define or suggest required
levelsofperformanceofbonecement.Thisfatiguetestmethod radius or taper between the specimen ends and gage section is
essential to reduce variation in reported fatigue life. Suggested
isnotintendedtorepresenttheclinicaluseoforthopaedicbone
cement, but rather to characterize the material using standard specimen dimensions are provided in Fig. 1.
and well-established methods. The user is cautioned to con-
sider the appropriateness of this test method in view of the 8. Specimen Preparation
material being tested and its potential application.
8.1 Cement Mixing:
5.4 It is widely reported that multiple clinical factors affect 8.1.1 Store the liquid and powder portions of the cement
the fatigue performance of orthopaedic bone cement; however, according to the manufacturer’s instructions before mixing.
the actual mechanisms involves multiple factors. Clinical 8.1.2 Allow the mixing equipment to equilibrate to room
factors which may affect the performance of bone cement temperaturebeforemixing.Recordtheroomtemperatureatthe
include: temperature and humidity, mixing method, time of onset of mixing.
application, surgical technique, bone preparation, implant 8.1.3 Mix the powder and liquid components according to
design,anatomicalsite,andpatientfactors,amongothers.This the manufacturer’s instructions and begin recording the time
test method does not specifically address all of these clinical using a stopwatch when the liquid and powder are initially
factors. The test method can be used to compare different mixed. Report any deviations from the manufacturer’s storage
acrylic bone cement formulations and products and different and mixing recommendations.
F2118 − 14 (2020)
FIG. 1 Specimen Dimensions
8.1.4 Report the mixing method and any equipment used. as a surface discontinuity greater than 250 mm in major
The method used for mixing the cement may affect its fatigue diameter. All specimens should be photographed to document
behavior. See X2.13 for further information. surface finish prior to testing. In addition, the specimens’
straightness should be compared to the metal positive blank to
8.2 Specimen Fabrication—The cylindrical reduced gage
ensure that the specimen is will not product bending moments
section test specimens are fabricated using the following
duringtheuniaxialfatiguetesting.Straightnesscanbeassessed
method:
by rolling the specimens and determining if there is a visible
8.2.1 Direct Molding:
wobbleascomparedtothestraightmetallicblankusedtomake
8.2.1.1 Inject the mixed cement into a specimen mold
the mold. Specimens with surface defects or deemed not to be
during the dough phase as determined by Specification F451
straight shall be rejected from testing and discarded. The total
(manufactured from silicone material, see Appendix X3 (sug-
number of specimens rejected divided by the total number of
gested specimen molding method)) with an internal cavity
specimens manufactured (rejection rate) shall be reported. A
which has the same dimensions as the final cement test
rationale for these rejection criteria is provided in X2.11.
specimen.Recordthemethodofcementinsertionintothemold
(that is, syringe-injected). A 150 mL syringe with an inner
8.4 Specimen Finishing—If necessary, lightly polish the
diameter of 38 mm and a nozzle tip diameter of 10 mm should
gage length of the specimens with 600-grit abrasive paper in
be considered for use. The mold should be placed on a flat
the longitudinal direction until the surface is free of machining
surface.The cement injection should be performed from top to
and/or mold marks. It should be noted that molds can wear
bottom in direction allowing the cement to flow down axially
over time as they are used, and a visual inspection of the
to the bottom . The bottom of the mold is placed on a flat
surface roughness of each specimen should be done to ensure
surface as the bone cement is being injected into the mold
smoothness. New molds should be made when the smoothness
unixaially from the top down. If air is entrapped and leads to
can no longer be achieved with light polish.
resistance to injection, the mold should be rocked back and
8.5 Specimen Measurement—Measure the diameter of the
forth to release trapped air from the bottom of the mold. This
specimens at a minimum of three places along the gage length
will allow for air to escape from the bottom of the mold. (See
of each specimen.The average of these measurements shall be
X3.6forstandardoperatingprocedureformakingbonecement
used as the specimen’s gage diameter for calculation of the
specimens.)
required load.
8.2.1.2 Place the mold in a container of phosphate buffered
saline (PBS). The PBS solution should be maintained at 37 6
8.6 Specimen Conditioning:
2°C.After at least1hinthePBS bath, the specimens may be
8.6.1 PlacethetestspecimensinPBSwhichismaintainedat
removed from the mold. Appendix X3 describes a suggested
a temperature of 37 6 2°C.
procedure for molding cement specimens.
8.6.2 Maintain the specimens in the PBS solution for a
8.3 Specimen Examination: minimumof7days.Thecementspecimensshallbemaintained
8.3.1 Visually examine specimens for surface defects. Sur- in the PBS solution for 7 to 60 days. The specimens shall be
face defects in the gage or transition sections (radii) shall be continuallyimmersedinthetestsolutionsothattheydonotdry
rejected from testing and discarded.Asurface defect is defined out. Distilled water shall be added to the soaking chamber
F2118 − 14 (2020)
during the soaking period to make up for evaporation loss. investigated should be considered when determining the ap-
Each specimen should be soaked up to the time immediately propriate sample size; while this may require more than 15
before its being mounted on the load frame. See X2.5 for specimensperbonecementformulationateachstresslevel,15
further information. is the recommended minimum number to test. See X2.12 for
further information.
9. Fatigue Test Procedures
9.6 Set the cycle counter and limit settings of the test frame
9.1 Mount one specimen at a time in a test frame test such controllertorecordthecumulativenumberofcyclesappliedto
that a uniaxial load is applied. Collets, Jacob’s chucks, or
the test specimen and the appropriate test limits values to
pressurized grips should be used to firmly grip the specimen at indicate specimen
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.