ASTM D5134-98(2003)
(Test Method)Standard Test Method for Detailed Analysis of Petroleum Naphthas through n-Nonane by Capillary Gas Chromatography
Standard Test Method for Detailed Analysis of Petroleum Naphthas through <i>n</i>-Nonane by Capillary Gas Chromatography
SIGNIFICANCE AND USE
A knowledge of the hydrocarbon components comprising a petroleum naphtha, reformate, or alkylate is useful in valuation of crude oils, in alkylation and reforming process control, in product quality assessment, and for regulatory purposes. Detailed hydrocarbon composition is also used as input in the mathematical modeling of refinery processes.
Separation of naphtha components by the procedure described in this test method can result in some peaks that represent coeluting compounds. This test method cannot attribute relative concentrations to the coelutants. In the absence of supporting information, use of the results of this test method for purposes which require such attribution is not recommended.
SCOPE
1.1 This test method covers the determination of hydrocarbon components of petroleum naphthas as enumerated in Table 1. Components eluting after n-nonane (bp 150.8°C) are determined as a single group.
1.2 This test method is applicable to olefin-free (
1.3 Components that are present at the 0.05 mass % level or greater can be determined.
1.4 The values stated in SI units are to be regarded as the standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific warning statements are given in Section 7.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation:D5134–98 (Reapproved 2003)
Standard Test Method for
Detailed Analysis of Petroleum Naphthas through n-Nonane
by Capillary Gas Chromatography
This standard is issued under the fixed designation D5134; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
Despite the many advances in capillary gas chromatography instrumentation and the remarkable
resolution achievable, it has proven difficult to standardize a test method for the analysis of a mixture
as complex as petroleum naphtha. Because of the proliferation of numerous, similar columns and the
endless choices of phase thickness, column internal diameter, length, etc., as well as instrument
operating parameters, many laboratories use similar but not identical methods for the capillary GC
analysis of petroleum naphthas. Even minute differences in column polarity or column oven
temperature, for example, can change resolution or elution order of components and make their
identification an individual interpretive process rather than the desirable, objective application of
standard retention data. To avoid this, stringent column specifications and temperature and flow
conditions have been adopted in this test method to ensure consistent elution order and resolution and
reproducible retention times. Strict adherence to the specified conditions is essential to the successful
application of this test method.
1. Scope 2. Referenced Documents
1.1 This test method covers the determination of hydrocar- 2.1 ASTM Standards:
boncomponentsofpetroleumnaphthasasenumeratedinTable D1319 Test Method for Hydrocarbon Types in Liquid
1. Components eluting after n-nonane (bp 150.8°C) are deter- Petroleum Products by Fluorescent Indicator Adsorption
mined as a single group. D3700 Practice for Obtaining LPG Samples Using a Float-
1.2 This test method is applicable to olefin-free (<2% ing Piston Cylinder
olefins by liquid volume) liquid hydrocarbon mixtures includ- D3710 Test Method for Boiling Range Distribution of
ing virgin naphthas, reformates, and alkylates. Olefin content GasolineandGasolineFractionsbyGasChromatography
can be determined by Test Method D1319. The hydrocarbon D4057 Practice for Manual Sampling of Petroleum and
mixturemusthavea98%pointof250°Corlessasdetermined Petroleum Products
by Test Method D3710.
3. Summary of Test Method
1.3 Componentsthatarepresentatthe0.05mass%levelor
greater can be determined. 3.1 A representative sample of the naphtha is introduced
into a gas chromatograph equipped with a methyl silicone
1.4 The values stated in SI units are to be regarded as the
bondedphasefusedsilicacapillarycolumn.Heliumcarriergas
standard.
1.5 This standard does not purport to address all of the transports the vaporized sample through the column in which
the components are separated. Components are sensed by a
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro- flame ionization detector as they elute from the column. The
detector signal is processed by an electronic data acquisition
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. Specific warning system or integrating computer. Each eluting peak is identified
by comparing its retention index to a table of retention indices
statements are given in Section 7.
and by visual matching with a standard chromatogram. The
table of retention indices has been established by running
This test method is under the jurisdiction of ASTM Committee D02 on
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
D02.04 on Hydrocarbon Analysis.
Annual Book of ASTM Standards, Vol 05.01.
Current edition approved May 10, 2003. Published August 2003. Originally
Annual Book of ASTM Standards, Vol 05.02.
approved in 1990. Last previous edition approved in 1998 as D5134–98.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D5134–98 (2003)
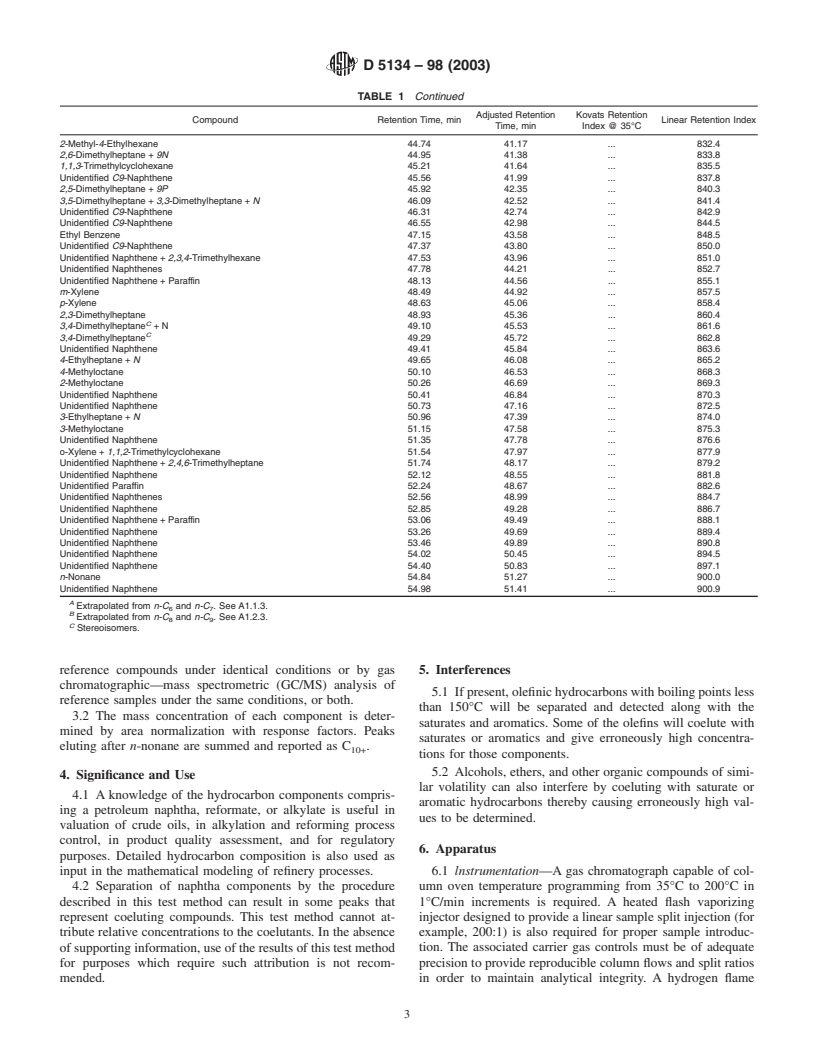
TABLE 1 Typical Retention Characteristics of Naphtha Components
NOTE—The abbreviations N and P refer to unidentified naphthenes and paraffins respectively.
Adjusted Retention Kovats Retention
Compound Retention Time, min Linear Retention Index
Time, min Index @ 35°C
Methane 3.57 0.00 100.0 .
Ethane 3.65 0.08 200.0 .
Propane 3.84 0.27 300.0 .
Isobutane 4.14 0.57 367.3 .
n-Butane 4.39 0.82 400.0 .
2,2-Dimethylpropane 4.53 0.96 415.5 .
Isopentane 5.33 1.76 475.0 .
n-Pentane 5.84 2.27 500.0 .
2,2-Dimethylbutane 6.81 3.24 536.2 .
Cyclopentane 7.83 4.26 564.1 .
2,3-Dimethylbutane 7.89 4.32 565.5 .
2-Methylpentane 8.06 4.49 569.5 .
3-Methylpentane 8.72 5.15 583.4 .
n-Hexane 9.63 6.06 600.0 .
2,2-Dimethylpentane 11.22 7.65 624.2 .
Methylcyclopentane 11.39 7.82 626.5 .
2,4-Dimethylpentane 11.68 8.11 630.3 .
2,2,3-Trimethylbutane 12.09 8.52 635.4 .
Benzene 13.29 9.72 649.1 .
3,3-dimethylpentane 13.84 10.27 654.8 .
Cyclohexane 14.19 10.62 658.3 .
2-Methylhexane 15.20 11.63 667.8 .
2,3-Dimethylpentane 15.35 11.78 669.1 .
1,1-Dimethylcyclopentane 15.61 12.04 671.4 .
3-Methylhexane 16.18 12.61 676.2 .
cis-1,3-Dimethylcyclopentane 16.88 13.31 681.8 .
trans-1,3-Dimethylcyclopentane 17.22 13.65 684.4 .
3-Ethylpentane 17.44 13.87 686.1 .
trans-1,2-Dimethylcyclopentane 17.57 14.00 687.0 .
2,2,4-Trimethylpentane 17.80 14.23 688.7 .
n-Heptane 19.43 15.86 700.0 .
A
Methylcyclohexane + cis-1,2-Dimethylcyclopentane 22.53 18.96 718.6 .
A
1,1,3-Trimethylcyclopentane + 2,2-Dimethylhexane 23.05 19.48 721.4 .
A
Ethylcyclopentane 24.59 21.02 729.3 .
A
2,5-Dimethylhexane + 2,2,3-Trimethylpentane 25.12 21.55 731.9 .
A
2,4-Dimethylhexane 25.47 21.90 733.5 .
A
1,trans-2,cis-4-Trimethylcyclopentane 26.43 22.86 738.0 .
A
3,3-Dimethylhexane 26.79 23.22 739.6 .
A
1,trans-2,cis-3-Trimethylcyclopentane 28.01 24.44 744.9 .
A
2,3,4-Trimethylpentane 28.70 25.13 747.8 .
A B
Toluene + 2,3,3-Trimethylpentane 29.49 25.92 751.1 730.2
B
1,1,2-Trimethylcyclopentane 31.21 27.64 . 741.7
B
2,3-Dimethylhexane 31.49 27.92 . 743.6
A
2-Methyl-3-ethylpentane 31.69 28.12 . 744.9
B
2-Methylheptane 33.06 29.49 . 754.1
B
4-Methylheptane + 3-Methyl-3-ethylpentane 33.34 29.77 . 756.0
B
3,4-Dimethylhexane 33.49 29.92 . 757.0
B
1,cis-2,trans-4-Trimethylcyclopentane + 1,cis-2,cis-4-Trimethylcyclopentane 33.73 30.16 . 758.6
B
cis-1,3-Dimethylcyclohexane 34.45 30.88 . 763.4
B
3-Methylheptane + 1,cis-2,trans-3-Trimethylcyclopentane 34.64 31.07 . 764.7
B
3-Ethylhexane + trans-1,4-Dimethylcyclohexane 34.83 31.26 . 766.0
B
1,1-Dimethylcyclohexane 35.81 32.24 . 772.5
B
2,2,5-Trimethylhexane + trans-1,3-Ethylmethylcyclopentane 36.75 33.18 . 778.8
B
cis-1,3-Ethylmethylcyclopentane 37.14 33.57 . 781.4
B
trans-1,2-Ethylmethylcyclopentane 37.39 33.82 . 783.1
B
2,2,4-Trimethylhexane + 1,1-Ethylmethylcyclopentane 37.68 34.11 . 785.1
B
trans-1,2-Dimethylcylohexane 38.14 34.57 . 788.1
B
1,cis-2,cis-3-Trimethylcyclopentane 39.21 35.64 . 795.3
trans-1,3-Dimethylcyclohexane + cis-1,4-Dimethylcyclohexane 39.54 35.97 . 797.5
n-Octane 39.91 36.34 . 800.0
Isopropylcyclopentane + 2,4,4-Trimethylhexane 40.76 37.19 . 805.7
Unidentified C9-Naphthene 40.88 37.31 . 806.5
Unidentified C8-Naphthene 41.52 37.95 . 810.8
Unidentified C9-Naphthene 41.88 38.31 . 813.2
cis-1,2-Ethylmethylcyclopentane + 2,3,5-Trimethylhexane 42.55 38.98 . 817.7
2,2-Dimethylheptane 43.20 39.63 . 822.0
cis-1,2-Dimethylcyclohexane 43.43 39.86 . 823.6
2,2,3-Trimethylhexane + 9N 43.76 40.19 . 825.8
2,4-Dimethylheptane 43.88 40.31 . 826.6
4,4-Dimethylheptane + 9N 44.09 40.52 . 828.0
Ethylcyclohexane + n-Propylcyclopentane 44.36 40.79 . 829.8
D5134–98 (2003)
TABLE 1 Continued
Adjusted Retention Kovats Retention
Compound Retention Time, min Linear Retention Index
Time, min Index @ 35°C
2-Methyl-4-Ethylhexane 44.74 41.17 . 832.4
2,6-Dimethylheptane + 9N 44.95 41.38 . 833.8
1,1,3-Trimethylcyclohexane 45.21 41.64 . 835.5
Unidentified C9-Naphthene 45.56 41.99 . 837.8
2,5-Dimethylheptane + 9P 45.92 42.35 . 840.3
3,5-Dimethylheptane + 3,3-Dimethylheptane + N 46.09 42.52 . 841.4
Unidentified C9-Naphthene 46.31 42.74 . 842.9
Unidentified C9-Naphthene 46.55 42.98 . 844.5
Ethyl Benzene 47.15 43.58 . 848.5
Unidentified C9-Naphthene 47.37 43.80 . 850.0
Unidentified Naphthene + 2,3,4-Trimethylhexane 47.53 43.96 . 851.0
Unidentified Naphthenes 47.78 44.21 . 852.7
Unidentified Naphthene + Paraffin 48.13 44.56 . 855.1
m-Xylene 48.49 44.92 . 857.5
p-Xylene 48.63 45.06 . 858.4
2,3-Dimethylheptane 48.93 45.36 . 860.4
C
3,4-Dimethylheptane + N 49.10 45.53 . 861.6
C
3,4-Dimethylheptane 49.29 45.72 . 862.8
Unidentified Naphthene 49.41 45.84 . 863.6
4-Ethylheptane + N 49.65 46.08 . 865.2
4-Methyloctane 50.10 46.53 . 868.3
2-Methyloctane 50.26 46.69 . 869.3
Unidentified Naphthene 50.41 46.84 . 870.3
Unidentified Naphthene 50.73 47.16 . 872.5
3-Ethylheptane + N 50.96 47.39 . 874.0
3-Methyloctane 51.15 47.58 . 875.3
Unidentified Naphthene 51.35 47.78 . 876.6
o-Xylene + 1,1,2-Trimethylcyclohexane 51.54 47.97 . 877.9
Unidentified Naphthene + 2,4,6-Trimethylheptane 51.74 48.17 . 879.2
Unidentified Naphthene 52.12 48.55 . 881.8
Unidentified Paraffin 52.24 48.67 . 882.6
Unidentified Naphthenes 52.56 48.99 . 884.7
Unidentified Naphthene 52.85 49.28 . 886.7
Unidentified Naphthene + Paraffin 53.06 49.49 . 888.1
Unidentified Naphthene 53.26 49.69 . 889.4
Unidentified Naphthene 53.46 49.89 . 890.8
Unidentified Naphthene 54.02 50.45 . 894.5
Unidentified Naphthene 54.40 50.83 . 897.1
n-Nonane 54.84 51.27 . 900.0
Unidentified Naphthene 54.98 51.41 . 900.9
A
Extrapolated from n-C and n-C . See A1.1.3.
6 7
B
Extrapolated from n-C and n-C . See A1.2.3.
8 9
C
Stereoisomers.
reference compounds under identical conditions or by gas 5. Interferences
chromatographic—mass spectrometric (GC/MS) analysis of
5.1 Ifpresent,olefinichydrocarbonswithboilingpointsless
reference samples under the same conditions, or both.
than 150°C will be separated and detected along with the
3.2 The mass concentration of each component is deter-
saturates and aromatics. Some of the olefins will coelute with
mined by area normalization with response factors. Peaks
saturates or aromatics and give erroneously high concentra-
eluting after n-nonane are summed and reported as C .
10+
tions for those components.
5.2 Alcohols, ethers, and other organic compounds of simi-
4. Significance and Use
lar volatility can also interfere by coeluting with saturate or
4.1 A knowledge of the hydrocarbon components compris-
aromatic hydrocarbons thereby causing erroneously high val-
ing a petroleum naphtha, reformate, or alkylate is useful in
ues to be determined.
valuation of crude oils, in alkylation and reforming process
control, in product quality assessment, and for regulatory
6. Apparatus
purposes. Detailed hydrocarbon composition is also used as
input in the mathematical modeling of refinery processes. 6.1 lnstrumentation—A gas chromatograph capable of col-
4.2 Separation of naphtha components by the procedure umn oven temperature programming from 35°C to 200°C in
described in this test method can result in some peaks that 1°C/min increments is required. A heated flash vaporizing
represent coeluting compounds. This test method cannot at- injector designed to provide a linear sample split injection (for
tribute relative concentrations to the coelutants. In the absence example, 200:1) is also required for proper sample introduc-
ofsupportinginformation,useoftheresultsofthistestmethod tion. The associated carrier gas controls must be of adequate
for purposes which require such attribution is not recom- precision to provide reproducible column flows and split ratios
mended. in order to maintain analytical integrity. A hydrogen flame
D5134–98 (2003)
ionization detector designed for optimum response with capil- 7.10 Toluene, 99+mol %. (Warning—Flammable. Vapor
lary columns (with the required gas controls and electronics) harmful.)
must meet or exceed the following specifications: 7.11 2,3,3-Trimethylpentane, 99+ mol %. (Warning—
Extremely flammable. Harmful if inhaled.)
Operating temperature 100°C to 300°C
Sensitivity >0.015 C/g
7.12 Column Evaluation Mixture, a qualitative synthetic
−12
Minimum detectability 5 3 10 g carbon/second
mixture of pure liquid hydrocarbons with the following ap-
Linearity >10
proximate composition: 0.5% toluene, 1% n-heptane, 1%
6.2 Sample Introduction System—Manual or automatic liq-
2,3,3-trimethylpentane, 1 % 2-methylheptane, 1 %
uid syringe sample injection to the splitting injector may be
4-methylheptane, 1% n-octane in 2-methylpentane solvent.
employed. Devices capable of 0.2 µL to 1.0 µL injections are 5
7.13 Reference Alkylate, actual refinery alkylation product
suitable. It should be noted that inadequate splitter design or
used to prepare Fig. 1. (Warning—Extremely flammable.
poor injection technique, or both, can result in sample frac-
Harmful if inhaled.)
tionation. Operating conditions which preclude fractionation
7.14 Reference Naphtha, actual refinery stream used to
should be determined in accordance with Section 11.
prepare Fig. 2. (Warning—Extremely flammable. Harmful if
6.3 Electronic Data Acquisition System—Any data acquisi-
inhaled.)
tion and integration device used for quantitation of these 5
7.15 Reference Reformate, actual refinery reformer prod-
analyses must meet or exceed these minimum requirements:
uct used to prepare Fig. 3. (Warning—Extremely flammable.
6.3.1 Capacity for at least 250 peaks/analysis.
Harmful if inhaled.)
6.3.2 Normalized area percent calculation with response
factors.
8. Sampling
6.3.3 Identification of individual components by retention
8.1 Hydrocarbon liquids (including naphthas) with Reid
time.
vapor pressures of 110 kPa (16 psi) or less may be sampled
6.3.4 Noise and spike rejection capability.
either into a floating piston cylinder or into an open container.
6.3.5 Sampling rates for fast (<1 s) peaks.
8.1.1 Cylinder Sampling—Refer toTest Method D3700 for
6.3.6 Positive and negative sloping baseline correction.
instructionsontransferringarepresentativesampleofahydro-
6.3.7 Peakdetectionsensitivityfornarrowandbroadpeaks.
carbon fluid from a source into a floating piston cylinder.Add
6.3.8 Perpendicular drop and tangent sk
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.