ASTM D6239-09(2015)
(Test Method)Standard Test Method for Uranium in Drinking Water by High-Resolution Alpha-Liquid-Scintillation Spectrometry (Withdrawn 2024)
Standard Test Method for Uranium in Drinking Water by High-Resolution Alpha-Liquid-Scintillation Spectrometry (Withdrawn 2024)
SIGNIFICANCE AND USE
5.1 This test method is a fast, cost-effective method that can yield limited isotopic activity levels for 238U and 234U, as well as total uranium activity. Although 232U is incorporated as a tracer, uranium recoveries for this test measured during the developmental work on this test method were usually between 95 and 105%.
5.2 The high-resolution alpha-liquid-scintillation spectrometer offers a constant (99.6 ± 0.1) % counting efficiency and instrument backgrounds as low as 0.001 counts per minute (min–1 ) over a 4 to 7 MeV energy range according to McDowell and McDowell (2). Count rates for extractive scintillator blanks and reagent blanks usually range from 0.01 min–1 to 0.1 min–1.
SCOPE
1.1 This test method covers determining the total soluble uranium activity in drinking water in the range of 0.037 Bq/L (1 pCi/L) or greater by selective solvent extraction and high-resolution alpha-liquid-scintillation spectrometry. The energy resolution obtainable with this technique also allows estimation of the 238U to 234U activity ratio.
1.2 This test method was tested successfully with reagent water and drinking water. It is the user's responsibility to ensure the validity of this test method for waters of untested matrices.
1.3 The values stated in SI units are to be regarded as standard. The values given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see Section 9.
WITHDRAWN RATIONALE
This test method covers determining the total soluble uranium activity in drinking water in the range of 0.037 Bq/L (1 pCi/L) or greater by selective solvent extraction and high-resolution alpha-liquid-scintillation spectrometry. The energy resolution obtainable with this technique also allows estimation of the 238U to 234U activity ratio.
Formerly under the jurisdiction of Committee D19 on Water, this test method was withdrawn in January 2024 in accordance with section 10.6.3 of the Regulations Governing ASTM Technical Committees, which requires that standards shall be updated by the end of the eighth year since the last approval date.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D6239 − 09 (Reapproved 2015)
Standard Test Method for
Uranium in Drinking Water by High-Resolution Alpha-Liquid-
Scintillation Spectrometry
This standard is issued under the fixed designation D6239; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D7282Practice for Set-up, Calibration, and Quality Control
of Instruments Used for Radioactivity Measurements
1.1 This test method covers determining the total soluble
uranium activity in drinking water in the range of 0.037 Bq/L
3. Terminology
(1 pCi/L) or greater by selective solvent extraction and
3.1 Definitions:
high-resolutionalpha-liquid-scintillationspectrometry.Theen-
3.1.1 Fordefinitionsoftermsusedinthistestmethod,refer
ergy resolution obtainable with this technique also allows
238 234 to Terminology D1129. For terms not included in this
estimation of the Uto U activity ratio.
reference, refer to other published glossaries (1).
1.2 This test method was tested successfully with reagent
water and drinking water. It is the user’s responsibility to 4. Summary of Test Method
ensure the validity of this test method for waters of untested
4.1 This test method is based on solvent extraction technol-
matrices.
ogy to isolate and concentrate uranium in drinking water for
1.3 The values stated in SI units are to be regarded as
counting via a high-resolution alpha-liquid-scintillation spec-
standard. The values given in parentheses are for information trometer.
only.
4.2 To determine total uranium, as well as limited isotopic
238 234
1.4 This standard does not purport to address all of the
uranium ( U and U) by activity in drinking water, a
safety concerns, if any, associated with its use. It is the
200–mLacidified water sample is first spiked with Uasan
responsibility of the user of this standard to establish appro-
isotopic tracer, boiled briefly to remove radon, and evaporated
priate safety and health practices and determine the applica-
until less than 50 mL remain. The solution is then made
bility of regulatory limitations prior to use. For specific hazard
approximately 0.01 M in diethylenetriaminepentaacetic acid
statements, see Section 9.
(DTPA) and the pH is adjusted to between 2.5 and 3.0. The
sample is transferred to a separatory funnel and equilibrated
2. Referenced Documents
with 1.50 mL of an extractive scintillator containing a dialkyl
phosphoric acid extracting agent. Under these conditions only
2.1 ASTM Standards:
uraniumisquantitativelytransferredtotheorganicphasewhile
D1129Terminology Relating to Water
the extraction of undesired ions is masked by the presence of
D1193Specification for Reagent Water
DTPA. Following phase separation, 1.00 mL of the organic
D2777Practice for Determination of Precision and Bias of
phase is sparged with dry argon gas to remove oxygen, a
Applicable Test Methods of Committee D19 on Water
chemical quench agent, and counted on a high-resolution
D3370Practices for Sampling Water from Closed Conduits
alpha-liquid-scintillation spectrometer and multichannel ana-
D3648Practices for the Measurement of Radioactivity
lyzer (MCA).
D5847Practice for Writing Quality Control Specifications
for Standard Test Methods for Water Analysis
4.3 The alpha spectrum of a sample that contains natural
uranium and that is analyzed with an internal U tracer will
appear similar to the spectrum in Fig. 1. An approximate
resolution of 250 keV FWHM for U (4.2 MeV) allows
This test method is under the jurisdiction ofASTM Committee D19 on Water
238 234 232
andisthedirectresponsibilityofSubcommitteeD19.04onMethodsofRadiochemi-
resolution and analysis of the U, U, and U energy
cal Analysis.
spectrum peaks when their activities are of the same order of
Current edition approved Jan. 1, 2015. Published January 2015. Originally
magnitude.Resolutionofthe U(4.4MeV)alphapeakisnot
approved in 1998. Last previous edition approved in 2009 as D6239–09. DOI:
10.1520/D6239-09R15. possible, but its activity, which accounts for approximately
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The boldface numbers in parenthesis refer to the list of references at the end of
the ASTM website. the text.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6239 − 09 (2015)
3+
30 mg of Fe did not interfere with the extraction of uranium
when the DTPA concentration was 0.010 M, and as much as
3+
250mgofFe didnotinterferewhentheDTPAconcentration
was increased to 0.10 M.As much as 2000 mg of calcium ion
2+
(Ca ) did not present an interference in a 0.010 M DTPA
2-
solution. Sulfate ion (SO ) did not interfere with the
extraction of uranium at concentrations as high as 1 M, but
−
hydrogen oxalate (HC O ) concentrations greater than 0.001
2 4
−
M and dihydrogen phosphate (H PO ) concentrations greater
2 4
than 0.2 M resulted in decreased uranium recovery. These
concentrations, however, are several orders of magnitude
higher than the normal concentration of these ions in drinking
water.
6.2 Beta- and gamma-emitting radionuclide interference is
minimized (typically 99.95% rejection of beta/gamma pulses)
according to McDowell and McDowell (2) by the pulse-shape
discrimination of the high-resolution alpha-liquid-scintillation
FIG. 1 Alpha Energy Spectrum of Natural Uranium and U
Tracer Measured on a High-Resolution Alpha-Liquid-Scintillation
spectrometer.
Spectrometer
6.3 Quenching, often a problem with liquid scintillation
counting, is significantly reduced by the use of extractive
2.2% of the total natural uranium activity, is included in the
scintillator technology and will only result in a normally
238 234
totaluraniumactivitycalculatedwhenthe Uand Upeaks
insignificantspectralenergyshiftwiththisprocedure.Noalpha
238 234
are in the region of interest (ROI). When the U and U
counts will be lost due to quenching.
peaks are integrated separately, a portion of the U activity
234 238 232
238 234 6.4 Uand Umayexistinthe Utracer.Theextentof
will be included in the U activity and the rest in the U
the positive bias should be determined periodically.
activity, depending on the exact ROIs selected. Likewise, if
236 233
present, U and U will not be resolved by the spectrom-
7. Apparatus
eter; however, their activity will be included in the total
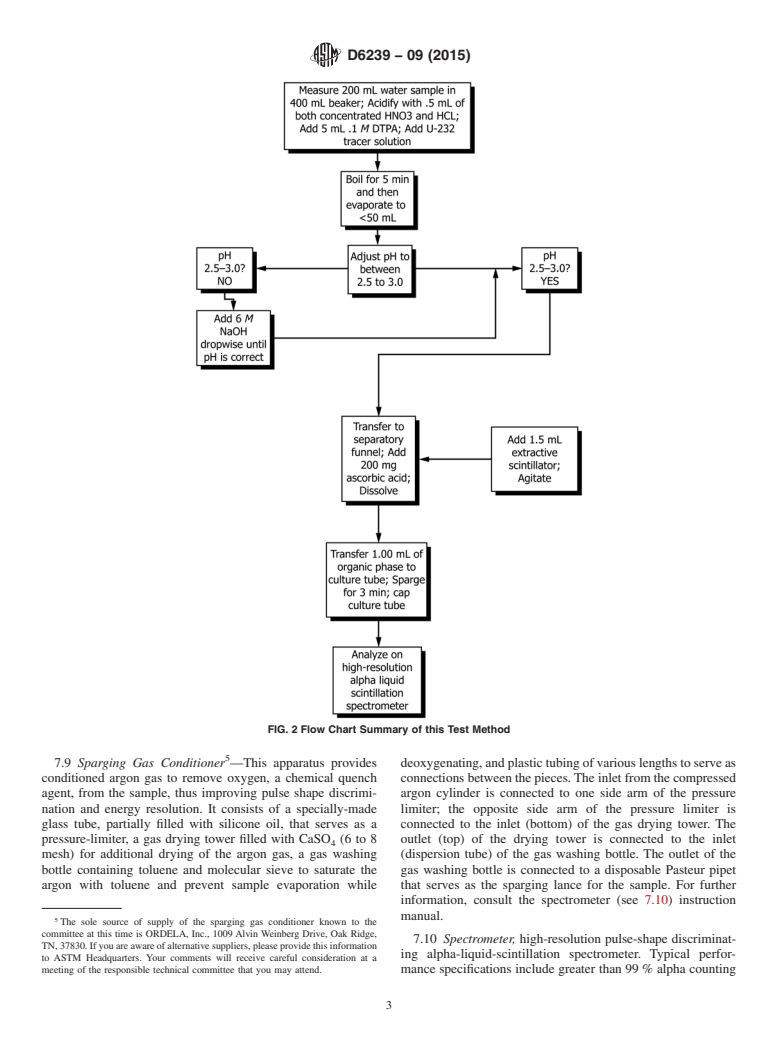
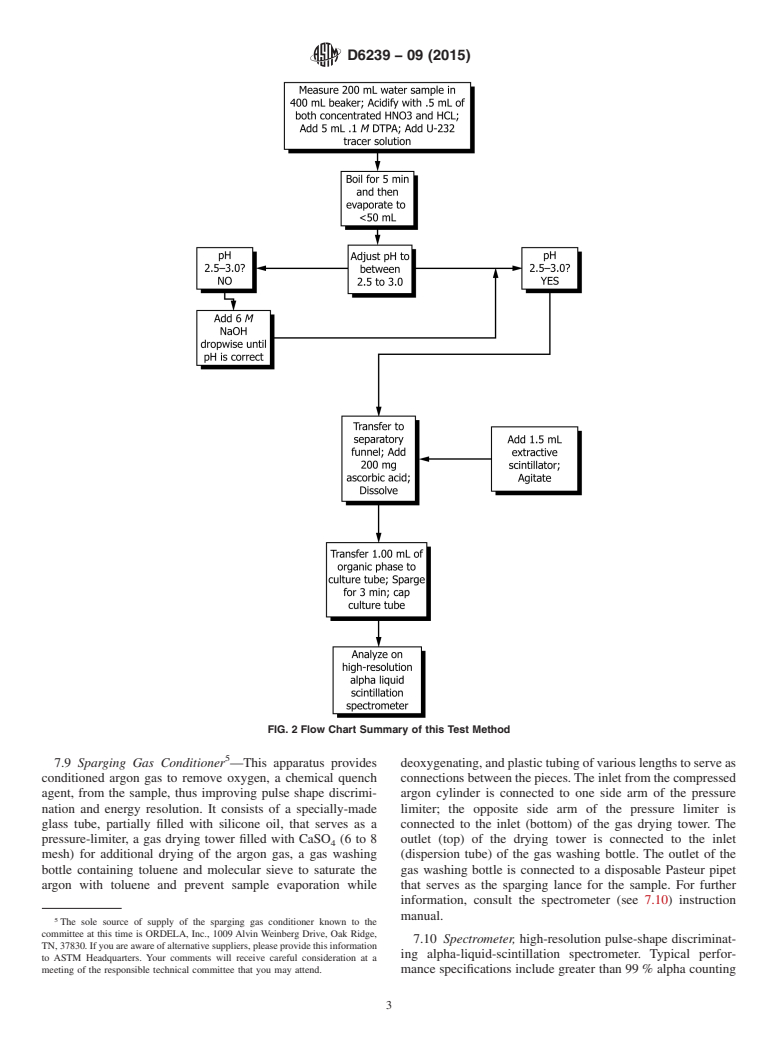
uranium ROI. Fig. 2 is a flow chart that summarizes the steps 7.1 Caps, vinyl or cork for culture tubes.
required in this test method.
7.2 Funnels, separatory, 125-mL, pear-shaped, polytetra-
fluoroethylene or polypropylene.
5. Significance and Use
7.3 Meter, pH, with gel electrode or low leak-rate reference
5.1 Thistestmethodisafast,cost-effectivemethodthatcan
238 234 electrode.
yield limited isotopic activity levels for U and U, as well
as total uranium activity. Although U is incorporated as a 7.4 Multichannel Analyzer (MCA), 512 channels or more,
tracer, uranium recoveries for this test measured during the ADC/memory or better.
developmental work on this test method were usually between
7.5 NIM Bin and Power Supply.
95 and 105%.
7.6 Power Supply, high voltage (+1000 V at 1 mA), or
5.2 The high-resolution alpha-liquid-scintillation spectrom-
integral to the spectrometer, see item 7.10.
eter offers a constant (99.6 6 0.1)% counting efficiency and
7.7 Sample, counting reference, normal uranium. This
instrument backgrounds as low as 0.001 counts per minute
–1
counting reference sample is an approximately 50/50 mix of
(min )overa4to7MeV energy range according to
238 234
U and U by activity in 1.00 mL of the extractive
McDowell and McDowell (2). Count rates for extractive
scintillator solution and enclosed in a 10 by 75 mm glass
scintillator blanks and reagent blanks usually range from 0.01
–1 –1 culture tube and is for standardization purposes only.
min to 0.1 min .
137 5
7.8 Source, Cs, approximately 1.85 × 10 Bq (5 µCi).
6. Interferences
This item is for standardization purposes only.
6.1 During the development work on this method, less than
241 238 210 226 222 230
1% of Am, Pu, Po, Ra, Rn, and Th present in
the original sample were found to extract under the conditions
described for the extraction of uranium by this procedure.
UraniumextractionisquantitativeatpHvaluesfrom1.0to5.0
230 238
but extraction of Th and Pu increased slightly at pH
4 238 234
values below 2.5 and phase separation was slower and less
The sole source of supply of the U and U normal uranium counting
reference sample known to the committee at this time is from ORDELA, Inc., 1009
complete at pH values above 3.5. DTPA concentration is not
Alvin Weinberg Drive, Oak Ridge, TN, 37830. If you are aware of alternative
critical in the range of 0.001 M to 0.1 M as long as a
suppliers, please provide this information to ASTM Headquarters. Your comments
stoichiometric excess relative to the concentration of interfer-
will receive careful consideration at a meeting of the responsible technical
3+
ingions,especiallyferricion(Fe ),ismaintained.Asmuchas committee that you may attend.
D6239 − 09 (2015)
FIG. 2 Flow Chart Summary of this Test Method
7.9 Sparging Gas Conditioner —This apparatus provides deoxygenating,andplastictubingofvariouslengthstoserveas
conditioned argon gas to remove oxygen, a chemical quench connectionsbetweenthepieces.Theinletfromthecompressed
agent, from the sample, thus improving pulse shape discrimi- argon cylinder is connected to one side arm of the pressure
nation and energy resolution. It consists of a specially-made limiter; the opposite side arm of the pressure limiter is
glass tube, partially filled with silicone oil, that serves as a connected to the inlet (bottom) of the gas drying tower. The
pressure-limiter, a gas drying tower filled with CaSO (6 to 8 outlet (top) of the drying tower is connected to the inlet
mesh) for additional drying of the argon gas, a gas washing (dispersion tube) of the gas washing bottle. The outlet of the
bottle containing toluene and molecular sieve to saturate the gas washing bottle is connected to a disposable Pasteur pipet
argon with toluene and prevent sample evaporation while that serves as the sparging lance for the sample. For further
information, consult the spectrometer (see 7.10) instruction
manual.
The sole source of supply of the sparging gas conditioner known to the
committee at this time is ORDELA, Inc., 1009Alvin Weinberg Drive, Oak Ridge,
7.10 Spectrometer, high-resolution pulse-shape discriminat-
TN,37830.Ifyouareawareofalternativesuppliers,pleaseprovidethisinformation
ing alpha-liquid-scintillation spectrometer. Typical perfor-
to ASTM Headquarters. Your comments will receive careful consideration at a
meeting of the responsible technical committee that you may attend. mance specifications include greater than 99% alpha counting
D6239 − 09 (2015)
efficiency, 99.95% beta/gamma rejection, energy resolution of 9.2 When diluting concentrated acids, always use safety
200to250keVFWHMforthe4.78MeV Raspectrumpeak glasses and protective clothing, and add the acid to the water.
and instrument backgrounds of 0.001 counts per minute over a
9.3 Tolueneisflammable.Avoidbreathingvapors.Usewith
4 to 7 MeV energy range.
adequate ventilation and avoid open flames.
7.11 Tubes, 10 by 75 mm borosilicate glass. These tubes
10. Sampling
serve as sample-counting cells for the spectrometer (see 7.10).
10.1 Collect the sample in accordance with the applicable
8. Reagents and Materials
methods as described in Practice D3370.
8.1 Purity of Reagents—Reagent grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
11. Calibration and Standardization
all reagents shall conform to the specifications of the Commit-
11.1 Use a normal uranium counting reference sample (that
tee onAnalytical Reagents of theAmerican Chemical Society
238 234
consists of an approximate 50/50 mixture of U and U, by
(3). Other grades may be used, provided it is first ascertained
activity) to establish an initial region of interest (ROI) on the
that the reagent is of sufficiently high purity to permit its use
multichannel analyzer (MCA).
without lessening the accuracy of the determination.
NOTE1—TheactualROIforanygivensamplemaydifferslightlyfrom
8.2 Purity of Water—Unless otherwise indicated, references
this initial ROI setting depending on the nature of the sample and the
towatershallbeunderstoodtomeanreagentwaterconforming
extractive scintillator used. This reference sample may be made using the
to Specification D1193, Type III, or better.
techniques cited in Burnett and Tai (5). Set the pulse shape discriminator
(PSD) of the high-resolution alpha-liquid-scintillation spectrometer prior
8.3 Argon Gas, Compressed—99.999% pure, with two-
5 137
to counting each individual sample.A1.85 × 10 Bq (5 microcurie) Cs
stage pressure regulator.
gamma source may be used to aid in setting the PSD by quickly inducing
abeta/gammapeak (4).Foradditionalinformation,refertotheinstrument
8.4 Ascorbic Acid— Reagent grade, solid ascorbic acid
instruction manual.
(C H O ).
6 8 6
NOTE 2—Setting the pulse shape discriminator (PSD) is a quick, but
criticalprocedure.InaccurateactivitydeterminationswillresultifthePSD
8.5 Dialkyl Phosphoric Acid Extractive Scintillator—See
is set improperly.
Ref (4).
11.2 Areagentblankispreparedwithouttracerforuseinthe
8.6 Diethylenetriaminepentaacetic Acid (DTPA)(0.1 M)—
background subtraction count (BSC). The reagent blank used
Add 3.93 g of solid DTPA(C H N O ) to 50 mL of water.
14 23 3 10
for the BSC must closely match the associated sample test
Adjust the pH approximately 7 by the dropwise addition of 6
source configuration to ensure that the measurements used for
M sodium hydroxide (NaOH) while stirring to complete
background subtraction accurately reflect conditions when
dissolution. Dilute to 100 mL with water.
countingsampletestsources.RefertoPracticeD7282,Section
8.7 Hydrochloric Acid (sp gr 1.19)—Concentrated hydro-
12.1.3.
chloric acid (HCl).
11.3 For general guidance on calibration and
8.8 Molecular Sieve—Type 4A, activated, indicating, 4-8
standardization, refer to Practice D3648.
mesh (Na [AlO ) (S
...
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D6239 − 09 (Reapproved 2015)
Standard Test Method for
Uranium in Drinking Water by High-Resolution Alpha-Liquid-
Scintillation Spectrometry
This standard is issued under the fixed designation D6239; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D7282 Practice for Set-up, Calibration, and Quality Control
of Instruments Used for Radioactivity Measurements
1.1 This test method covers determining the total soluble
uranium activity in drinking water in the range of 0.037 Bq/L
3. Terminology
(1 pCi/L) or greater by selective solvent extraction and
3.1 Definitions:
high-resolution alpha-liquid-scintillation spectrometry. The en-
3.1.1 For definitions of terms used in this test method, refer
ergy resolution obtainable with this technique also allows
238 234
to Terminology D1129. For terms not included in this
estimation of the U to U activity ratio.
reference, refer to other published glossaries (1).
1.2 This test method was tested successfully with reagent
water and drinking water. It is the user’s responsibility to
4. Summary of Test Method
ensure the validity of this test method for waters of untested
4.1 This test method is based on solvent extraction technol-
matrices.
ogy to isolate and concentrate uranium in drinking water for
1.3 The values stated in SI units are to be regarded as counting via a high-resolution alpha-liquid-scintillation spec-
standard. The values given in parentheses are for information
trometer.
only.
4.2 To determine total uranium, as well as limited isotopic
238 234
1.4 This standard does not purport to address all of the
uranium ( U and U) by activity in drinking water, a
safety concerns, if any, associated with its use. It is the
200–mL acidified water sample is first spiked with U as an
responsibility of the user of this standard to establish appro-
isotopic tracer, boiled briefly to remove radon, and evaporated
priate safety and health practices and determine the applica-
until less than 50 mL remain. The solution is then made
bility of regulatory limitations prior to use. For specific hazard
approximately 0.01 M in diethylenetriaminepentaacetic acid
statements, see Section 9.
(DTPA) and the pH is adjusted to between 2.5 and 3.0. The
sample is transferred to a separatory funnel and equilibrated
2. Referenced Documents
with 1.50 mL of an extractive scintillator containing a dialkyl
phosphoric acid extracting agent. Under these conditions only
2.1 ASTM Standards:
uranium is quantitatively transferred to the organic phase while
D1129 Terminology Relating to Water
the extraction of undesired ions is masked by the presence of
D1193 Specification for Reagent Water
DTPA. Following phase separation, 1.00 mL of the organic
D2777 Practice for Determination of Precision and Bias of
phase is sparged with dry argon gas to remove oxygen, a
Applicable Test Methods of Committee D19 on Water
chemical quench agent, and counted on a high-resolution
D3370 Practices for Sampling Water from Closed Conduits
alpha-liquid-scintillation spectrometer and multichannel ana-
D3648 Practices for the Measurement of Radioactivity
lyzer (MCA).
D5847 Practice for Writing Quality Control Specifications
for Standard Test Methods for Water Analysis
4.3 The alpha spectrum of a sample that contains natural
uranium and that is analyzed with an internal U tracer will
appear similar to the spectrum in Fig. 1. An approximate
resolution of 250 keV FWHM for U (4.2 MeV) allows
This test method is under the jurisdiction of ASTM Committee D19 on Water
238 234 232
and is the direct responsibility of Subcommittee D19.04 on Methods of Radiochemi-
resolution and analysis of the U, U, and U energy
cal Analysis.
spectrum peaks when their activities are of the same order of
Current edition approved Jan. 1, 2015. Published January 2015. Originally
magnitude. Resolution of the U (4.4 MeV) alpha peak is not
approved in 1998. Last previous edition approved in 2009 as D6239 – 09. DOI:
10.1520/D6239-09R15. possible, but its activity, which accounts for approximately
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The boldface numbers in parenthesis refer to the list of references at the end of
the ASTM website. the text.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6239 − 09 (2015)
3+
30 mg of Fe did not interfere with the extraction of uranium
when the DTPA concentration was 0.010 M, and as much as
3+
250 mg of Fe did not interfere when the DTPA concentration
was increased to 0.10 M. As much as 2000 mg of calcium ion
2+
(Ca ) did not present an interference in a 0.010 M DTPA
2-
solution. Sulfate ion (SO ) did not interfere with the
extraction of uranium at concentrations as high as 1 M, but
−
hydrogen oxalate (HC O ) concentrations greater than 0.001
2 4
−
M and dihydrogen phosphate (H PO ) concentrations greater
2 4
than 0.2 M resulted in decreased uranium recovery. These
concentrations, however, are several orders of magnitude
higher than the normal concentration of these ions in drinking
water.
6.2 Beta- and gamma-emitting radionuclide interference is
minimized (typically 99.95 % rejection of beta/gamma pulses)
according to McDowell and McDowell (2) by the pulse-shape
discrimination of the high-resolution alpha-liquid-scintillation
FIG. 1 Alpha Energy Spectrum of Natural Uranium and U
Tracer Measured on a High-Resolution Alpha-Liquid-Scintillation
spectrometer.
Spectrometer
6.3 Quenching, often a problem with liquid scintillation
counting, is significantly reduced by the use of extractive
2.2 % of the total natural uranium activity, is included in the
scintillator technology and will only result in a normally
238 234
total uranium activity calculated when the U and U peaks
insignificant spectral energy shift with this procedure. No alpha
238 234
are in the region of interest (ROI). When the U and U
counts will be lost due to quenching.
peaks are integrated separately, a portion of the U activity
234 238 232
238 234
6.4 U and U may exist in the U tracer. The extent of
will be included in the U activity and the rest in the U
the positive bias should be determined periodically.
activity, depending on the exact ROIs selected. Likewise, if
236 233
present, U and U will not be resolved by the spectrom-
7. Apparatus
eter; however, their activity will be included in the total
uranium ROI. Fig. 2 is a flow chart that summarizes the steps 7.1 Caps, vinyl or cork for culture tubes.
required in this test method.
7.2 Funnels, separatory, 125-mL, pear-shaped, polytetra-
fluoroethylene or polypropylene.
5. Significance and Use
7.3 Meter, pH, with gel electrode or low leak-rate reference
5.1 This test method is a fast, cost-effective method that can
238 234 electrode.
yield limited isotopic activity levels for U and U, as well
as total uranium activity. Although U is incorporated as a 7.4 Multichannel Analyzer (MCA), 512 channels or more,
tracer, uranium recoveries for this test measured during the ADC/memory or better.
developmental work on this test method were usually between
7.5 NIM Bin and Power Supply.
95 and 105%.
7.6 Power Supply, high voltage (+1000 V at 1 mA), or
5.2 The high-resolution alpha-liquid-scintillation spectrom-
integral to the spectrometer, see item 7.10.
eter offers a constant (99.6 6 0.1) % counting efficiency and
7.7 Sample, counting reference, normal uranium. This
instrument backgrounds as low as 0.001 counts per minute
–1
counting reference sample is an approximately 50/50 mix of
(min ) over a 4 to 7 MeV energy range according to
238 234
U and U by activity in 1.00 mL of the extractive
McDowell and McDowell (2). Count rates for extractive
scintillator solution and enclosed in a 10 by 75 mm glass
scintillator blanks and reagent blanks usually range from 0.01
–1 –1
culture tube and is for standardization purposes only.
min to 0.1 min .
137 5
7.8 Source, Cs, approximately 1.85 × 10 Bq (5 µCi).
6. Interferences
This item is for standardization purposes only.
6.1 During the development work on this method, less than
241 238 210 226 222 230
1% of Am, Pu, Po, Ra, Rn, and Th present in
the original sample were found to extract under the conditions
described for the extraction of uranium by this procedure.
Uranium extraction is quantitative at pH values from 1.0 to 5.0
230 238
but extraction of Th and Pu increased slightly at pH
4 238 234
values below 2.5 and phase separation was slower and less
The sole source of supply of the U and U normal uranium counting
reference sample known to the committee at this time is from ORDELA, Inc., 1009
complete at pH values above 3.5. DTPA concentration is not
Alvin Weinberg Drive, Oak Ridge, TN, 37830. If you are aware of alternative
critical in the range of 0.001 M to 0.1 M as long as a
suppliers, please provide this information to ASTM Headquarters. Your comments
stoichiometric excess relative to the concentration of interfer-
will receive careful consideration at a meeting of the responsible technical
3+
ing ions, especially ferric ion (Fe ), is maintained. As much as committee that you may attend.
D6239 − 09 (2015)
FIG. 2 Flow Chart Summary of this Test Method
7.9 Sparging Gas Conditioner —This apparatus provides deoxygenating, and plastic tubing of various lengths to serve as
conditioned argon gas to remove oxygen, a chemical quench connections between the pieces. The inlet from the compressed
agent, from the sample, thus improving pulse shape discrimi- argon cylinder is connected to one side arm of the pressure
nation and energy resolution. It consists of a specially-made limiter; the opposite side arm of the pressure limiter is
glass tube, partially filled with silicone oil, that serves as a connected to the inlet (bottom) of the gas drying tower. The
pressure-limiter, a gas drying tower filled with CaSO (6 to 8 outlet (top) of the drying tower is connected to the inlet
mesh) for additional drying of the argon gas, a gas washing (dispersion tube) of the gas washing bottle. The outlet of the
bottle containing toluene and molecular sieve to saturate the gas washing bottle is connected to a disposable Pasteur pipet
argon with toluene and prevent sample evaporation while that serves as the sparging lance for the sample. For further
information, consult the spectrometer (see 7.10) instruction
manual.
The sole source of supply of the sparging gas conditioner known to the
committee at this time is ORDELA, Inc., 1009 Alvin Weinberg Drive, Oak Ridge,
7.10 Spectrometer, high-resolution pulse-shape discriminat-
TN, 37830. If you are aware of alternative suppliers, please provide this information
ing alpha-liquid-scintillation spectrometer. Typical perfor-
to ASTM Headquarters. Your comments will receive careful consideration at a
meeting of the responsible technical committee that you may attend. mance specifications include greater than 99 % alpha counting
D6239 − 09 (2015)
efficiency, 99.95 % beta/gamma rejection, energy resolution of 9.2 When diluting concentrated acids, always use safety
200 to 250 keV FWHM for the 4.78 MeV Ra spectrum peak glasses and protective clothing, and add the acid to the water.
and instrument backgrounds of 0.001 counts per minute over a
9.3 Toluene is flammable. Avoid breathing vapors. Use with
4 to 7 MeV energy range.
adequate ventilation and avoid open flames.
7.11 Tubes, 10 by 75 mm borosilicate glass. These tubes
10. Sampling
serve as sample-counting cells for the spectrometer (see 7.10).
10.1 Collect the sample in accordance with the applicable
8. Reagents and Materials
methods as described in Practice D3370.
8.1 Purity of Reagents—Reagent grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
11. Calibration and Standardization
all reagents shall conform to the specifications of the Commit-
11.1 Use a normal uranium counting reference sample (that
tee on Analytical Reagents of the American Chemical Society
238 234
consists of an approximate 50/50 mixture of U and U, by
(3). Other grades may be used, provided it is first ascertained
activity) to establish an initial region of interest (ROI) on the
that the reagent is of sufficiently high purity to permit its use
multichannel analyzer (MCA).
without lessening the accuracy of the determination.
NOTE 1—The actual ROI for any given sample may differ slightly from
8.2 Purity of Water—Unless otherwise indicated, references
this initial ROI setting depending on the nature of the sample and the
to water shall be understood to mean reagent water conforming
extractive scintillator used. This reference sample may be made using the
to Specification D1193, Type III, or better.
techniques cited in Burnett and Tai (5). Set the pulse shape discriminator
(PSD) of the high-resolution alpha-liquid-scintillation spectrometer prior
8.3 Argon Gas, Compressed—99.999 % pure, with two-
5 137
to counting each individual sample. A 1.85 × 10 Bq (5 microcurie) Cs
stage pressure regulator.
gamma source may be used to aid in setting the PSD by quickly inducing
a beta/gamma peak (4). For additional information, refer to the instrument
8.4 Ascorbic Acid— Reagent grade, solid ascorbic acid
instruction manual.
(C H O ).
6 8 6
NOTE 2—Setting the pulse shape discriminator (PSD) is a quick, but
critical procedure. Inaccurate activity determinations will result if the PSD
8.5 Dialkyl Phosphoric Acid Extractive Scintillator—See
is set improperly.
Ref (4).
11.2 A reagent blank is prepared without tracer for use in the
8.6 Diethylenetriaminepentaacetic Acid (DTPA)(0.1 M)—
background subtraction count (BSC). The reagent blank used
Add 3.93 g of solid DTPA (C H N O ) to 50 mL of water.
14 23 3 10
for the BSC must closely match the associated sample test
Adjust the pH approximately 7 by the dropwise addition of 6
source configuration to ensure that the measurements used for
M sodium hydroxide (NaOH) while stirring to complete
background subtraction accurately reflect conditions when
dissolution. Dilute to 100 mL with water.
counting sample test sources. Refer to Practice D7282, Section
8.7 Hydrochloric Acid (sp gr 1.19)—Concentrated hydro-
12.1.3.
chloric acid (HCl).
11.3 For general guidance on calibration and
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.