ASTM E882-10(2016)
(Guide)Standard Guide for Accountability and Quality Control in the Chemical Analysis Laboratory
Standard Guide for Accountability and Quality Control in the Chemical Analysis Laboratory
SIGNIFICANCE AND USE
4.1 An accountability and quality control system is established by laboratory management to improve the quality of its results. It provides documented records which serve to assure users of the laboratory's services that a specified level of precision is achieved in the routine performance of its measurements and that the data reported were obtained from the samples submitted. The system also provides for: early warning to analysts when methods or equipment begin to develop a bias or show deterioration of precision; the protection and retrievability of data (results); traceability and control of samples as they are processed through the laboratory; good communication of sample information between submitters, analysts, and supervision; and information on sample processing history. This guide describes such a system. Other accountability and quality control programs can be developed. Such programs can be equivalent to the program in this guide if they provide all of the benefits mentioned above.
SCOPE
1.1 This guide covers the essential aspects of an accountability and quality control program for a chemical analysis laboratory. The reasons for establishing and operating such a program are discussed.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E882 − 10 (Reapproved 2016)
Standard Guide for
Accountability and Quality Control in the Chemical Analysis
Laboratory
This standard is issued under the fixed designation E882; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope ing to analysts when methods or equipment begin to develop a
bias or show deterioration of precision; the protection and
1.1 This guide covers the essential aspects of an account-
retrievability of data (results); traceability and control of
ability and quality control program for a chemical analysis
samples as they are processed through the laboratory; good
laboratory. The reasons for establishing and operating such a
communication of sample information between submitters,
program are discussed.
analysts, and supervision; and information on sample process-
ing history.This guide describes such a system. Other account-
2. Referenced Documents
ability and quality control programs can be developed. Such
2.1 ASTM Standards:
programs can be equivalent to the program in this guide if they
E135 Terminology Relating to Analytical Chemistry for
provide all of the benefits mentioned above.
Metals, Ores, and Related Materials
E1329 Practice for Verification and Use of Control Charts in
5. Accountability
Spectrochemical Analysis
5.1 Accountability means assurance that the results reported
MNL 7A Manual on Presentation of Data and Control Chart
refer directly to the samples submitted.
Analysis
2.2 ASQC Document: 5.2 Prior to submitting samples to the laboratory, the pro-
ASQC Standard A1 Definitions, Symbols, Formulas, and spective user should consult with laboratory personnel con-
Tables for Control Charts cerning his needs and the capability of the laboratory to satisfy
them. It is the responsibility of the originator of the samples to
3. Terminology
select and identify proper samples for submission to the
laboratory, to decide what information is required, and, after
3.1 Definitions—For definitions of terms used in this guide,
consulting with laboratory personnel, to submit the samples in
refer to Terminology E135.
suitable containers, properly labeled, and accompanied by
4. Significance and Use written instructions identifying the samples, their nature, and
the information sought through chemical analysis. This should
4.1 An accountability and quality control system is estab-
be done formally, using a well-defined document for informa-
lished by laboratory management to improve the quality of its
tion transfer to initiate work in the laboratory.
results. It provides documented records which serve to assure
users of the laboratory’s services that a specified level of
5.3 Laboratory management establishes a written account-
precision is achieved in the routine performance of its mea- ability system to be used throughout the laboratory at all times.
surements and that the data reported were obtained from the
This implies traceability and documentation of all reported
samples submitted. The system also provides for: early warn- results through the laboratory back to the submitted sample.
This system should have the following general characteristics:
5.3.1 Each testing request submitted by a user of the
This guide is under the jurisdiction of ASTM Committee E01 on Analytical
laboratory’s services is assigned an internal laboratory identi-
Chemistry for Metals, Ores, and Related Materials and is the direct responsibility of
fication number (ID), which is used to correlate all samples,
Subcommittee E01.22 on Laboratory Quality.
Current edition approved Dec. 1, 2016. Published December 2016. Originally
work, time, and cost accounting, consultation, and reports and
approved in 1982. Last previous edition approved in 2010 as E882 – 10. DOI:
other paperwork associated with that request. The final report
10.1520/E0882-10R16.
that is returned to the originator will always bear the number
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
(ID) for future reference. Moreover, it is convenient for
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
laboratory data to be filed according to sequential ID numbers.
the ASTM website.
For example, “86/0428” might identify the associated work as
ASTM Manual Series, ASTM, 7th Edition, 2002.
the 428th request submitted in the year 1986.The Data Record
Available from American Society for Quality (ASQ), 600 N. Plankinton Ave.,
Milwaukee, WI 53203, http://www.asq.org. shouldprovidealldatageneratedduringtheanalyses,namesof
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E882 − 10 (2016)
persons performing the analyses, dates the analyses were 6.2.1 Involvetheoperatorsoranalystswhoactuallyperform
performed, and any unusual occurrences that happened during the work to the greatest possible extent.
the analyses. Accountability for production control samples is
6.2.2 Use the simplest, most direct statistical procedures
normally maintained separately from the other testing records that will provide the necessary degree of control. This means
because results from production control samples are usually thatgraphicalorsimplifiedarithmeticproceduresarepreferred.
reported on routine report forms, the samples being identified
6.2.3 Perform the quality control measurements as early in
with the day, shift, run, or lot from which they were taken.
the measurement process as possible. This prevents waste of
analytical effort if the method is not initially in control.
5.3.2 Each sample, specimen, sample site, or other unique
However, when a prolonged series of measurements is made, it
pieceofmaterialorcontaineridentifiedasaseparatesampleby
is also necessary to verify that the method remains in control
the originator should be assigned a sequential item number
throughout the run.
(NN) for internal laboratory use. As soon as the samples are
accepted by the laboratory, laboratory personnel will mark 6.2.4 Provide specific action limits and describe exactly
what must be done when these limits are exceeded.
each sample or sample container with its own laboratory
sample number (ID-NN) in such manner that the label is not 6.2.5 For each method (for each sample type), choose a
likely to become separated from its sample or rendered control material that is known to be stable, homogeneous and
unreadable during its residence in the laboratory. For example, has measured values within the range of interest.Any inhomo-
the fifth sample on the above-mentioned request might be geneity in the control sample will add to the variance of the
identified as “86/0428-05.” results. Any increase in variability that is not related to the
measurement process will reduce the sensitivity of the quality
5.3.3 All laboratory work records, intermediate sample
control procedure to detect changes in the measurement
containers, data, and reports for a specific sample will be
process. Where possible, the control material should be similar
identified by the same laboratory identification and item
to the samples to be analyzed. Obtain as large an amount of
number to avoid any opportunity for samples or data to be lost
control material as can be prepared in a homogeneous state
or intermixed within or between requests.
because considerable effort is required to prepare a new
5.3.4 The first and last steps in the accountability procedure
control.Alwaysprepareanewcontrolmaterialwellinadvance
are functions of technical supervision. Before any work is
of exhausting the old one so that the new supply is ready when
performed, the compatibility of the work requested with the
needed.Insituationswheresatisfactorycontrolmaterialcannot
physical condition of the samples and the capabilities of the
be obtained, alternative techniques (such as, retest by a senior
laboratory must be verified.When the analysts have completed
analyst) may be substituted for the control material approach.
their work, the results must be reviewed to be certain that all
6.2.6 Give analysts specific instructions concerning their
information requested has been determined and that the work
response to an out-of-control condition. Supervision may
has been performed with the required care and precision. In
decide that, if the analyst can correct the problem so that the
this latter regard, quality control procedures prove invaluable
control sample results are again within limits, the process may
both to the analysts performing the work and the reviewing
continue without immediate contact with the supervisor. In
supervisor. The supervisor also verifies that the results are
other situations, the supervisor may need to become involved
calculated in units that are most meaningful to the submitter
witheachout-of-controlincident.Ineithercase,adjustmentsto
and that the units and basis on which the results are calculated
the process should be recorded to explain each shift in the
are clearly stated.
control measurements.
5.3.5 Except for the most routine work, the original ana-
6.2.7 Provide for a periodic in-depth review by supervision
lyst’s data book, a serial listing of laboratory identification
and management of the overall effectiveness of the laboratory
numbers and descriptions, and a copy of each job report are
quality control system. Operating experience may indicate that
retained in the laboratory’s records for the periods of time
methodsshouldbeaddedto,ordroppedfromtheprogram,that
established by laboratory policy. Intermediate calculations and
the frequency of specific control samples should be increased
samples are normally discarded after the submitter has had a
or decreased, or that a different strategy might be more
reasonable opportunity to submit questions concerning the
appropriate for control of a specific method. The interval for
results and request return of his samples. In some cases,
such reviews should be determined by the uniformity of the
customer specifications may dictate the records that must be
processes that generate the samples. Any anticipated or ob-
retained and the retention times for both analytical records and
served change in the character of the samples being analyzed
laboratory samples.
should initiate at least a cursory review of the control proce-
dures for the methods that apply to those samples.
6. Quality Control
6.3 Laboratory Quality Control Strategies—Control chart
6.1 Quality control of analytical methods provides the
methods are suitable for laboratory quality control programs.
information needed to ensure that procedures, equipment, and
The choice of which control strategy to use depends on
personnel are performing at the levels of precision and accu-
circumstances: the type of instrument or laboratory procedure,
racy required by the intended use of the data.
the number of samples and frequency of the analyses, and the
closeness of control required. The following are appropriate:
6.2 General Characteristics—The following factors have
¯
been found helpful in maximizing the effectiveness and mini- 6.3.1 The X- and R-chart method is most frequently used.
mizing the cost of quality control procedures: The control sample is run two or more times during the run,
E882 − 10 (2016)
¯
batch, or shift. The average is plotted on the X-chart and the infrequently used analytical methods or for non-routine sample
absolute value of the difference between the high and low types. If a CRM (from the National Institute of Standards and
values, the range, is plotted on the R-chart. If the average falls Technology or other CRM producer) similar to the samples in
between the upper and lower control limits and the range falls composition is tested with the samples, comparison of the
belowtheuppercontrollimit,theprocessisconsideredtobein measured value to the assigned value of the CRM provides a
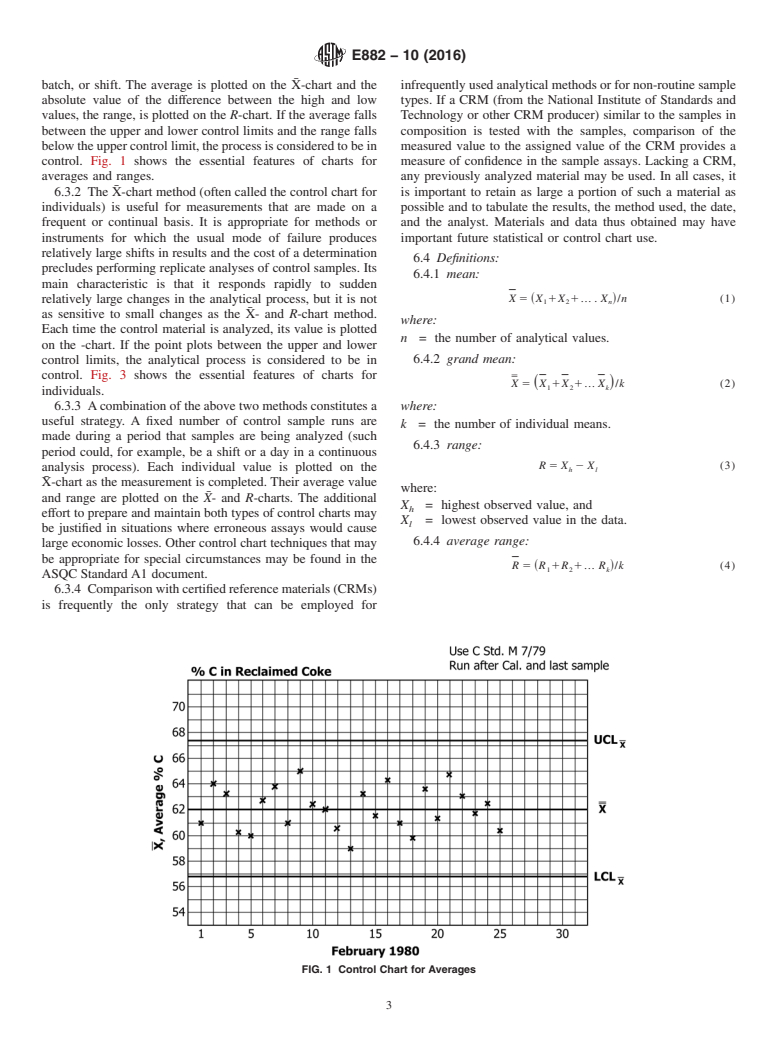
control. Fig. 1 shows the essential features of charts for measure of confidence in the sample assays. Lacking a CRM,
averages and ranges. any previously analyzed material may be used. In all cases, it
¯
6.3.2 The X-chart method (often called the control chart for is important to retain as large a portion of such a material as
individuals) is useful for measurements that are made on a possible and to tabulate the results, the method used, the date,
frequent or continual basis. It is appropriate for methods or and the analyst. Materials and data thus obtained may have
instruments for which the usual mode of failure produces important future statistical or control chart use.
relatively large shifts in results and the cost of a determination
6.4 Definitions:
precludes performing replicate analyses of control samples. Its
6.4.1 mean:
main characteristic is that it responds rapidly to sudden
H
X 5 X 1X 1…. X /n (1)
relatively large changes in the analytical process, but it is not ~ !
1 2 n
¯
as sensitive to small changes as the X- and R-chart method.
where:
Each time the control material is analyzed, its value is plotted
n = the number of analytical values.
on the -chart. If the point plots between the upper and lower
6.4.2 grand mean:
control limits, the analytical process is considered to be in
control. Fig. 3 shows the essential features of charts for
% H H H
X 5 ~X 1X 1… X !/k (2)
1 2 k
individuals.
6.3.3 Acombination of the above two methods constitutes a where:
useful strategy. A fixed number of control sample runs are
k = the number of individual means.
made during a period that samples are being analyzed (such
6.4.3 range:
period could, for example, be a shift or a day in a continuous
R 5 X 2 X (3)
analysis process). Each individual value is plotted on the
h l
¯
X-chart as the measurement is completed. Their average value
where:
¯
and range are plotted on the X- and R-charts. The additional
X = highest observed value, and
h
effort to prepare and maintain both types of control charts may
X = lowest observed value in the data.
l
be justified in situations where erroneous assays would cause
6.4.4 average range:
large economic losses. Other control chart techniques that may
be appropriate for special circumstances may be found in the
H
R 5 ~R 1R 1… R !/k (4)
1 2 k
ASQC Standard A1 document.
6.3.4 Comparisonwithcertifiedreferencematerials(CRMs)
is frequently the only strategy that can be employed for
FIG. 1 Control Chart for Averages
E882 − 10 (2016)
FIG. 2 Control Chart for Ranges
FIG. 3 Co
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E882 − 10 E882 − 10 (Reapproved 2016)
Standard Guide for
Accountability and Quality Control in the Chemical Analysis
Laboratory
This standard is issued under the fixed designation E882; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide covers the essential aspects of an accountability and quality control program for a chemical analysis laboratory.
The reasons for establishing and operating such a program are discussed.
2. Referenced Documents
2.1 ASTM Standards:
E135 Terminology Relating to Analytical Chemistry for Metals, Ores, and Related Materials
E1329 Practice for Verification and Use of Control Charts in Spectrochemical Analysis
MNL 7A Manual on Presentation of Data and Control Chart Analysis
2.2 ASQC Document:
ASQC Standard A1 Definitions, Symbols, Formulas, and Tables for Control Charts
3. Terminology
3.1 Definitions—For definitions of terms used in this guide, refer to Terminology E135.
4. Significance and Use
4.1 An accountability and quality control system is established by laboratory management to improve the quality of its results.
It provides documented records which serve to assure users of the laboratory’s services that a specified level of precision is
achieved in the routine performance of its measurements and that the data reported were obtained from the samples submitted. The
system also provides for: early warning to analysts when methods or equipment begin to develop a bias or show deterioration of
precision; the protection and retrievability of data (results); traceability and control of samples as they are processed through the
laboratory; good communication of sample information between submitters, analysts, and supervision; and information on sample
processing history. This guide describes such a system. Other accountability and quality control programs can be developed. Such
programs can be equivalent to the program in this guide if they provide all of the benefits mentioned above.
5. Accountability
5.1 Accountability means assurance that the results reported refer directly to the samples submitted.
5.2 Prior to submitting samples to the laboratory, the prospective user should consult with laboratory personnel concerning his
needs and the capability of the laboratory to satisfy them. It is the responsibility of the originator of the samples to select and
identify proper samples for submission to the laboratory, to decide what information is required, and, after consulting with
laboratory personnel, to submit the samples in suitable containers, properly labeled, and accompanied by written instructions
identifying the samples, their nature, and the information sought through chemical analysis. This should be done formally, using
a well-defined document for information transfer to initiate work in the laboratory.
This guide is under the jurisdiction of ASTM Committee E01 on Analytical Chemistry for Metals, Ores, and Related Materials and is the direct responsibility of
Subcommittee E01.22 on Laboratory Quality.
Current edition approved Oct. 1, 2010Dec. 1, 2016. Published December 2010 December 2016. Originally approved in 1982. Last previous edition approved in 20032010
as E882 – 98 (2003).E882 – 10. DOI: 10.1520/E0882-10.10.1520/E0882-10R16.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
ASTM Manual Series, ASTM, 7th Edition, 2002.
Available from American Society for Quality (ASQ), 600 N. Plankinton Ave., Milwaukee, WI 53203, http://www.asq.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E882 − 10 (2016)
5.3 Laboratory management establishes a written accountability system to be used throughout the laboratory at all times. This
implies traceability and documentation of all reported results through the laboratory back to the submitted sample. This system
should have the following general characteristics:
5.3.1 Each testing request submitted by a user of the laboratory’s services is assigned an internal laboratory identification
number (ID), which is used to correlate all samples, work, time, and cost accounting, consultation, and reports and other paperwork
associated with that request. The final report that is returned to the originator will always bear the number (ID) for future reference.
Moreover, it is convenient for laboratory data to be filed according to sequential ID numbers. For example, “86/0428” might
identify the associated work as the 428th request submitted in the year 1986. The Data Record should provide all data generated
during the analyses, names of persons performing the analyses, dates the analyses were performed, and any unusual occurrences
that happened during the analyses. Accountability for production control samples is normally maintained separately from the other
testing records because results from production control samples are usually reported on routine report forms, the samples being
identified with the day, shift, run, or lot from which they were taken.
5.3.2 Each sample, specimen, sample site, or other unique piece of material or container identified as a separate sample by the
originator should be assigned a sequential item number (NN) for internal laboratory use. As soon as the samples are accepted by
the laboratory, laboratory personnel will mark each sample or sample container with its own laboratory sample number (ID-NN)
in such manner that the label is not likely to become separated from its sample or rendered unreadable during its residence in the
laboratory. For example, the fifth sample on the above-mentioned request might be identified as “86/0428-05.”
5.3.3 All laboratory work records, intermediate sample containers, data, and reports for a specific sample will be identified by
the same laboratory identification and item number to avoid any opportunity for samples or data to be lost or intermixed within
or between requests.
5.3.4 The first and last steps in the accountability procedure are functions of technical supervision. Before any work is
performed, the compatibility of the work requested with the physical condition of the samples and the capabilities of the laboratory
must be verified. When the analysts have completed their work, the results must be reviewed to be certain that all information
requested has been determined and that the work has been performed with the required care and precision. In this latter regard,
quality control procedures prove invaluable both to the analysts performing the work and the reviewing supervisor. The supervisor
also verifies that the results are calculated in units that are most meaningful to the submitter and that the units and basis on which
the results are calculated are clearly stated.
5.3.5 Except for the most routine work, the original analyst’s data book, a serial listing of laboratory identification numbers and
descriptions, and a copy of each job report are retained in the laboratory’s records for the periods of time established by laboratory
policy. Intermediate calculations and samples are normally discarded after the submitter has had a reasonable opportunity to submit
questions concerning the results and request return of his samples. In some cases, customer specifications may dictate the records
that must be retained and the retention times for both analytical records and laboratory samples.
6. Quality Control
6.1 Quality control of analytical methods provides the information needed to ensure that procedures, equipment, and personnel
are performing at the levels of precision and accuracy required by the intended use of the data.
6.2 General Characteristics—The following factors have been found helpful in maximizing the effectiveness and minimizing
the cost of quality control procedures:
6.2.1 Involve the operators or analysts who actually perform the work to the greatest possible extent.
6.2.2 Use the simplest, most direct statistical procedures that will provide the necessary degree of control. This means that
graphical or simplified arithmetic procedures are preferred.
6.2.3 Perform the quality control measurements as early in the measurement process as possible. This prevents waste of
analytical effort if the method is not initially in control. However, when a prolonged series of measurements is made, it is also
necessary to verify that the method remains in control throughout the run.
6.2.4 Provide specific action limits and describe exactly what must be done when these limits are exceeded.
6.2.5 For each method (for each sample type), choose a control material that is known to be stable, homogeneous and has
measured values within the range of interest. Any inhomogeneity in the control sample will add to the variance of the results. Any
increase in variability that is not related to the measurement process will reduce the sensitivity of the quality control procedure to
detect changes in the measurement process. Where possible, the control material should be similar to the samples to be analyzed.
Obtain as large an amount of control material as can be prepared in a homogeneous state because considerable effort is required
to prepare a new control. Always prepare a new control material well in advance of exhausting the old one so that the new supply
is ready when needed. In situations where satisfactory control material cannot be obtained, alternative techniques (such as, retest
by a senior analyst) may be substituted for the control material approach.
6.2.6 Give analysts specific instructions concerning their response to an out-of-control condition. Supervision may decide that,
if the analyst can correct the problem so that the control sample results are again within limits, the process may continue without
immediate contact with the supervisor. In other situations, the supervisor may need to become involved with each out-of-control
incident. In either case, adjustments to the process should be recorded to explain each shift in the control measurements.
E882 − 10 (2016)
6.2.7 Provide for a periodic in-depth review by supervision and management of the overall effectiveness of the laboratory
quality control system. Operating experience may indicate that methods should be added to, or dropped from the program, that the
frequency of specific control samples should be increased or decreased, or that a different strategy might be more appropriate for
control of a specific method. The interval for such reviews should be determined by the uniformity of the processes that generate
the samples. Any anticipated or observed change in the character of the samples being analyzed should initiate at least a cursory
review of the control procedures for the methods that apply to those samples.
6.3 Laboratory Quality Control Strategies—Control chart methods are suitable for laboratory quality control programs. The
choice of which control strategy to use depends on circumstances: the type of instrument or laboratory procedure, the number of
samples and frequency of the analyses, and the closeness of control required. The following are appropriate:
6.3.1 The X¯- and R-chart method is most frequently used. The control sample is run two or more times during the run, batch,
or shift. The average is plotted on the X¯-chart and the absolute value of the difference between the high and low values, the range,
is plotted on the R-chart. If the average falls between the upper and lower control limits and the range falls below the upper control
limit, the process is considered to be in control. Fig. 1 shows the essential features of charts for averages and ranges.
6.3.2 The X¯-chart method (often called the control chart for individuals) is useful for measurements that are made on a frequent
or continual basis. It is appropriate for methods or instruments for which the usual mode of failure produces relatively large shifts
in results and the cost of a determination precludes performing replicate analyses of control samples. Its main characteristic is that
it responds rapidly to sudden relatively large changes in the analytical process, but it is not as sensitive to small changes as the
X¯- and R-chart method. Each time the control material is analyzed, its value is plotted on the X¯-chart. If the point plots between
the upper and lower control limits, the analytical process is considered to be in control. Fig. 3 shows the essential features of charts
for individuals.
6.3.3 A combination of the above two methods constitutes a useful strategy. A fixed number of control sample runs are made
during a period that samples are being analyzed (such period could, for example, be a shift or a day in a continuous analysis
process). Each individual value is plotted on the X¯-chart as the measurement is completed. Their average value and range are
plotted on the X¯- and R-charts. The additional effort to prepare and maintain both types of control charts may be justified in
situations where erroneous assays would cause large economic losses. Other control chart techniques that may be appropriate for
special circumstances may be found in the ASQC Standard A1 document.
6.3.4 Comparison with certified reference materials (CRMs) is frequently the only strategy that can be employed for
infrequently used analytical methods or for non-routine sample types. If a CRM (from the National Institute of Standards and
Technology or other CRM producer) similar to the samples in composition is tested with the samples, comparison of the measured
value to the assigned value of the CRM provides a measure of confidence in the sample assays. Lacking a CRM, any previously
analyzed material may be used. In all cases, it is important to retain as large a portion of such a material as possible and to tabulate
the results, the method used, the date, and the analyst. Materials and data thus obtained may have important future statistical or
control chart use.
6.4 Definitions:
6.4.1 mean:
FIG. 1 Control Chart for Averages
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.