ASTM C351-92b(1999)
(Test Method)Standard Test Method for Mean Specific Heat of Thermal Insulation
Standard Test Method for Mean Specific Heat of Thermal Insulation
SCOPE
1.1 This test method covers the determination of mean specific heat of thermal insulating materials. The materials must be essentially homogeneous and composed of matter in the solid state.
1.2 This test method employs the classical method of mixtures. This provides procedures and apparatus simpler than those generally used in scientific calorimetry, an accuracy that is adequate for most thermal insulating purposes, and a degree of precision that is reproducible by laboratory technicians of average skill. While this test method was developed for testing thermal insulations, it is easily adaptable to measuring the specific heat of other materials.
1.3 The test procedure provides for a mean temperature of approximately 60°C (100 to 20°C temperature range), using water as the calorimetric fluid. By substituting other calorimetric fluids the temperature range may be changed as desired.
1.4 The values stated in SI units are to be regarded as the standard.
1.5 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: C 351 – 92b (Reapproved 1999)
Standard Test Method for
Mean Specific Heat of Thermal Insulation
This standard is issued under the fixed designation C 351; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope being an average rather than a point value.) The unit of
measurement is J/kg·K.
1.1 This test method covers the determination of mean
3.1.2 thermal capacity—the amount of heat necessary to
specific heat of thermal insulating materials. The materials
change the temperature of the body one degree. For a homo-
must be essentially homogeneous and composed of matter in
geneous body it is the product of mass and specific heat. For a
the solid state.
nonhomogeneous body it is the sum of the products of mass
1.2 This test method employs the classical method of
and specific heat of the individual constituents. Thermal
mixtures. This provides procedures and apparatus simpler than
capacity has the units of J/K.
those generally used in scientific calorimetry, an accuracy that
3.1.3 thermal diffusivity—the ratio of thermal conductivity
is adequate for most thermal insulating purposes, and a degree
of a substance to the product of its density and specific heat.
of precision that is reproducible by laboratory technicians of
Common unit for this property is m /s.
average skill. While this test method was developed for testing
3.1.4 water equivalent—the mass of water that requires the
thermal insulations, it is easily adaptable to measuring the
same amount of heat as the given body in order to change its
specific heat of other materials.
temperature by an equal amount.
1.3 The test procedure provides for a mean temperature of
approximately 60°C (100 to 20°C temperature range), using
4. Summary of Test Method
water as the calorimetric fluid. By substituting other calorimet-
4.1 Themethodofmixturesusedinthistestmethodconsists
ric fluids the temperature range may be changed as desired.
essentiallyofaddingaknownmassofmaterialataknownhigh
1.4 The values stated in SI units are to be regarded as the
temperature to a known mass of water at a known low
standard.
temperature and determining the equilibrium temperature that
1.5 This standard does not purport to address all of the
results. The heat absorbed by the water and the containing
safety concerns, if any, associated with its use. It is the
vessel can be calculated and this value equated to the expres-
responsibility of the user of this standard to establish appro-
sion for the heat given up by the hot material. From this
priate safety and health practices and determine the applica-
equation the unknown specific heat can be calculated.
bility of regulatory limitations prior to use.
5. Significance and Use
2. Referenced Documents
5.1 Mean specific heat is an essential property of a thermal
2.1 ASTM Standards:
2 insulating material when the latter is used under conditions of
E1 Specification for ASTM Thermometers
unsteady or transient heat flow. It is a part of the parameter,
3. Terminology thermal diffusivity, which governs the rate of temperature
diffusion through insulation. It is a basic thermodynamic
3.1 Definitions:
property of all substances, the value of which depends upon
3.1.1 mean specific heat—the quantity of heat required to
chemical composition and temperature.
change the temperature of a unit mass of a substance one
degree, measured as the average quantity over the temperature
NOTE 1—Specific heat of insulations, as measured by this test method,
range specified. (It is distinguished from true specific heat by
using small specimens of a multi-component composite or of a low-
density product that has to be highly compressed, may not be directly
applicable for use in calculations involving transient thermal response.
The applicability of the results will depend upon a system being analyzed,
This test method is under the jurisdiction of ASTM Committee C-16 on the desired accuracy, and the relative amounts, and specific heats of the
Thermal Insulation and is the direct responsibility of Subcommittee C16.30 on
Thermal Measurements.
Current edition approved Aug. 15, 1992. Published October 1992. Originally
published as C 351 – 54 T. Last previous edition C 351 – 92a. Weber, R. L., Heat and Temperature Measurement, Prentice-Hall, New York,
Annual Book of ASTM Standards, Vol 14.03. NY, 1950.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
C 351 – 92b (1999)
various solid or fluid components, or both, of the thermal insulation.
brass pipe with asbestos paper, winding about 70 turns of
22-gage (0.64 mm) Nichrome wire over the paper, and insu-
6. Apparatus
lating the assembly with 25.4-mm thick pipe insulation. It is
6.1 The typical apparatus is shown schematically in Fig. 1.
necessary that the end turns be closer together than those over
It consists of the following:
thecenterportionoftheheatertocompensateforendheatloss.
6.1.1 Calorimeter and Accessories—The calorimeter shall
The heater temperature is controlled by regulating the electric
be an unlagged Dewar flask with a maximum capacity of not
current to the heater with a variable transformer or resistor. A
less than 500 mL nor more than 750 mL. The flask shall have
constant voltage source of power within 61 % maximum
an insulated cover or stopper. Other accessories shall include a
voltage variation is necessary to minimize temperature fluc-
magnetic stirrer equipped with a speed-regulating device.
tuations.
6.1.2 Differential Temperature Sensor—An appropriate
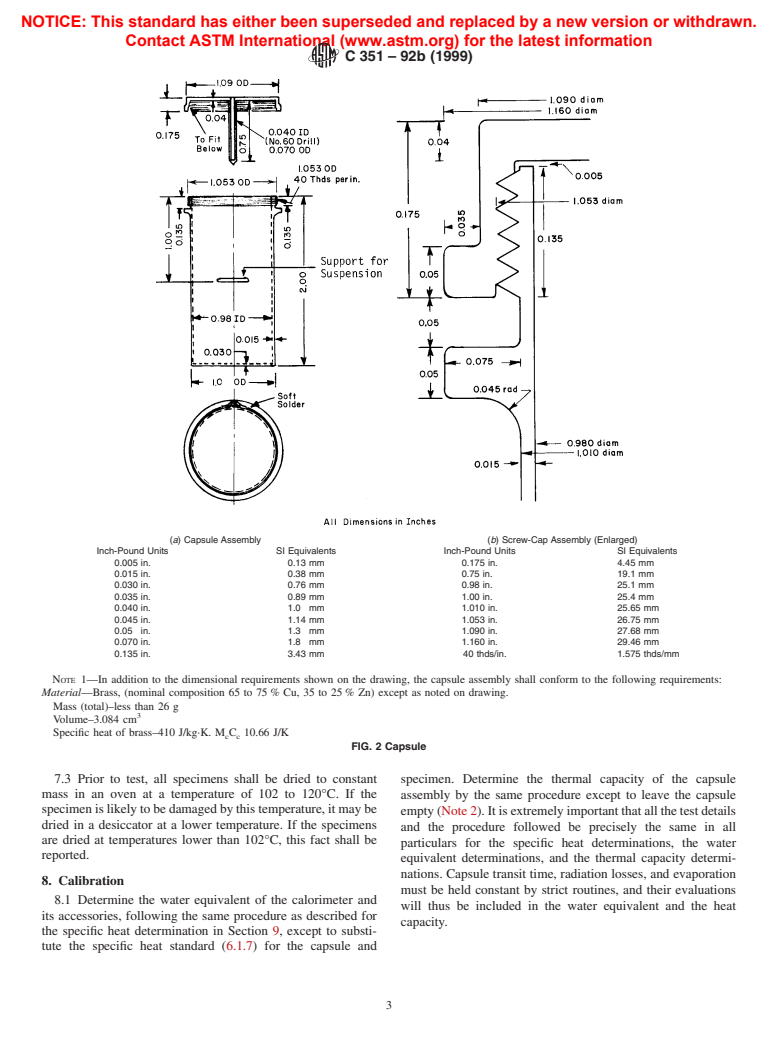
6.1.4 Capsule—The capsule shall comprise a hollow cylin-
temperature difference sensor, such as a Beckmann differential
der of brass approximately 25.4 mm in diameter by 50.8 mm
thermometer or a suitable equivalent, preferably with a mag-
long. It shall have a removable cap and a thermocouple well
nifier, and having a range of at least 5°C and a sensitivity of no
extending into the cavity space to accommodate the tempera-
less than 0.01°C shall be used to determine the rise in
ture sensor. It is imperative that the capsule assembly be
temperature of the calorimetric fluid during test. Where a
absolutely watertight, as no leakage can be tolerated. The
differential thermometer is used, it shall be set with its lowest
completed capsule, including cap, gasket, and suspension loop,
temperature division at the approximate room temperature, and
shallhaveathermalcapacitynotexceeding10.5J/K.Acapsule
the setting point checked with a precision-type temperature
design meeting the above requirements is shown in Fig. 2.
sensor (such as Thermometer 632C, preferably with a magni-
6.1.5 Temperature Sensor—A suitable, calibrated tempera-
fier, described in SpecificationE1).
turesensorandassociatedread-outequipmentofsuitablerange
6.1.3 Heater—The heater shall be of the open-end radiation
and precision to permit reading temperatures to an accuracy of
typesimilartothecylindricaldeviceshowninFig.1.Itmaybe
0.1°C shall be used. If a thermocouple is used, the wire size
heated by electricity or steam. The relative dimensions of the
should be small to limit the error caused by thermal conduc-
heater and the capsule shall be such that the specimen will be
tance losses along the length. Thermocouples can be made
heated to a uniform and constant temperature as required. A
from any of the standard pairs registered with the National
maximum variation of 6 1°C over the length of the heater is
Institute of Standards and Technology. A particular suitable
permitted. The heater shall be provided with an insulated
thermocouple is chromel/constantan, fabricated from wires
removable top cover designed both to permit passage of the
having a diameter no greater than No. 30 B & S gage (0.265
leadsofthetemperaturesensorandtosuspendthecapsule.The
mm). The pair combines the attributes of reduced heat leakage
bottom shall be closed with a removable insulated cover to
and a higher emf than copper/constantan.
permit free dropping of the capsule. The heater assembly shall
6.1.6 TestRoom—The temperature of the room in which the
be mounted so it can be swung quickly into place over the
test is conducted shall be reasonably constant during the test
calorimeter.
period. The test room temperature control is satisfactory if the
6.1.3.1 A convenient form of electric heater can be con-
time-temperature curve is a straight line (within the allowable
structed by covering a 254-mm length of 38-mm diameter
experimental error) for a 10-min period before the dropping of
thecapsuleandfora10-minperiodjustpriortothetermination
of the test.
6.1.7 Specific Heat Standard—Electrolytic copper (com-
mercial electrical bus bar copper) shall be used as a specific
heat standard.Astandard specimen as shown in Fig. 3 shall be
used to determine the water equivalent of the calorimeter flask
and its accessories. For the temperature range covered (be-
tween 100 and 20°C) the mean specific heat of copper shall be
taken as 390.0 J/(kg·K).
7. Test Specimens
7.1 Specimens shall be selected at random as required to
provide test material representative of the lot sampled. The
number of specimens may be determined by agreement but
shall be not less than three.
7.2 The specimens shall be tested in the compressed form,
since it is desirable to have as large a mass as possible. A
specimen press consisting of a hollow cylinder with a close-
fitting plunger used in conjunction with a bench vise or small
hydraulic press has been found convenient.
“Reference Table for Thermocouples,” Circular No. 508, National Institute of
FIG. 1 Specific Heat Calorimeter Standards and Technology, Gaithersburg, MD 20899.
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
C 351 – 92b (1999)
(a) Capsule Assembly (b) Screw-Cap Assembly (Enlarged)
Inch-Pound Units SI Equivalents Inch-Pound Units SI Equivalents
0.005 in. 0.13 mm 0.175 in. 4.45 mm
0.015 in. 0.38 mm 0.75 in. 19.1 mm
0.030 in. 0.76 mm 0.98 in. 25.1 mm
0.035 in. 0.89 mm 1.00 in. 25.4 mm
0.040 in. 1.0 mm 1.010 in. 25.65 mm
0.045 in. 1.14 mm 1.053 in. 26.75 mm
0.05 in. 1.3 mm 1.090 in. 27.68 mm
0.070 in. 1.8 mm 1.160 in. 29.46 mm
0.135 in. 3.43 mm 40 thds/in. 1.575 thds/mm
NOTE 1—In addition to the dimensional requirements shown on the drawing, the capsule assembly shall conform to the following requirements:
Material—Brass, (nominal composition 65 to 75 % Cu, 35 to 25 % Zn) except as noted on drawing.
Mass (total)–less than 26 g
Volume–3.084 cm
Specific heat of brass–410 J/kg·K. M C 10.66 J/K
c c
FIG. 2 Capsule
7.3 Prior to test, all specimens shall be dried to constant specimen. Determine the thermal capacity of the capsule
mass in an oven at a temperature of 102 to 120°C. If the assembly by the same procedure except to leave the capsule
specimenislikelytobedamagedbythistemperature,itmaybe
empty(Note2).Itisextremelyimportantthatallthetestdetails
dried in a desiccator at a lower temperature. If the specimens
and the procedure followed be precisely the same in all
are dried at temperatures lower than 102°C, this fact shall be
particulars for the specific heat determinations, the water
reported.
equivalent determinations, and the thermal capacity determi-
nations. Capsule transit time, radiation losses, and evaporation
8. Calibration
must be held constant by strict routines, and their evaluations
8.1 Determine the water equivalent of the calorimeter and
will thus be included in the water equivalent and the heat
its accessories, following the same procedure as described for
capacity.
the specific heat determination in Section 9, except to substi-
tute the specific heat standard (6.1.7) for the capsule and
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
C 351 – 92b (1999)
capsule is actually being dropped into the flask. It is also
important that a moderate stirring rate be used, note being
made of the speed setting, and care taken that the same setting
be used during all determinations. An appreciable amount of
heat is introduced by the agitation of the water. In order to
secure greatest accuracy, the rate of introduction of heat should
be as low as possible and particularly, as constant as possible.
NOTE 4—This test method specifies a temperature range of approxi-
mately 100°C to 20°C. When specific heat information is needed at other
temperatures, a similar test procedure should be followed. In general,
distilled water is suitable as a calorimetric fluid for capsule temperatures
up to 150°C. Where the upper limit of the temperature range is greater
than 150°C, a liquid with a high boiling point having a mean specific heat
(over the temperature range T −T ) known to an accuracy of 61 % and
m c
a flash point above the temperature of the heated capsule, T , must be
h
used. This liquid shall be noncorrosive to the apparatus, essentially
nontoxic, and nonirritating to the skin and should have a low vapor
Inch-Pound Units SI Equivalents Inch-Pound Units SI Equivalents
pressure to minimize volatilization losses. Where the lower limit of the
⁄8 in. 3.2 mm 2 in. 50.8 mm
temperature range is below 0°C, the calorimeter bath must be a low
⁄4 in. 6.4 mm No. 40 drill 6 mm
freezing point liquid having a mean specific heat (over the temperature
⁄2 in. 12.7 mm No. 55 drill 4 mm
rangeT −T ) known to an accuracy of 61 %. It must be noncorrosive to
1 in. 25.4 mm m c
the apparatus, essentially nontoxic, nonirritating to the skin, and resistant
FIG. 3 Specific Heat Standard
to hydrolysis.
9.3 After approximately 10 min, when a constant thermal
NOTE 2—It may be desirable to weight the empty capsule with a known
exchange exists between the calorimeter and the environment,
quantity of bus bar copper to facilitate the transfer to the calorimeter. If
start observations of the temperature of the calorimeter. Since
this is done it must be taken into account in the subsequent calculations.
the calorimeter is close to ambient temperature, the rate of
9. Procedure
thermal exchange will be small. Record the temperature of the
calorimeter (estimated to the nearest 0.001°C) at the end of
9.1 Place a dried specimen of as large a ma
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.