ASTM D5173-97(2007)
(Test Method)Standard Test Method for On-Line Monitoring of Carbon Compounds in Water by Chemical Oxidation, by UV Light Oxidation, by Both, or by High Temperature Combustion Followed by Gas Phase NDIR or by Electrolytic Conductivity
Standard Test Method for On-Line Monitoring of Carbon Compounds in Water by Chemical Oxidation, by UV Light Oxidation, by Both, or by High Temperature Combustion Followed by Gas Phase NDIR or by Electrolytic Conductivity
SIGNIFICANCE AND USE
Accurate measurement of organic carbon in water at low and very low levels is of particular interest to the electronic, pharmaceutical, and steam power generation industries.
Elevated levels of organics in raw water tend to degrade ion exchange resin capacity. Elevated levels of organics in high purity water tend to support biological growth and, in some cases, are directly detrimental to the processes that require high purity water.
In the case of steam power generation, naturally occurring organics can become degraded to CO2 and low molecular weight organic acids that, in turn, are corrosive to the process equipment. Their effect on conductivity may also cause water chemistry operating parameters to be exceeded, calling for plant shutdown.
In process water in other industries, organic carbon can signify in-leakage of substances through damaged piping and components, or an unacceptable level of product loss.
In wastewater treatment, organic carbon measurement of influent and in-process water can help adjust optimize treatment schemes. Measurement of organic carbon at discharge may contribute to regulatory compliance.
SCOPE
1.1 This test method covers the selection, establishment, and application of monitoring systems for carbon and carbon compounds by continual sampling or continuous flow-through, automatic analysis, and recording or otherwise signaling of output data. The system chosen will depend on the purpose for which it is intended (for example, regulatory compliance, process monitoring, or to alert the user to adverse trends) and on the type of water to be monitored (low purity or high purity, with or without suspended particulates, purgeable organics, or inorganic carbon). If it is to be used for regulatory compliance, the test method published or referenced in the regulations should be used in conjunction with this test method and other ASTM test methods. The test method covers carbon concentrations of 10 g/L to 5000 mg/L.
1.2 The values stated in SI units are to be regarded as the standard.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see Section 9.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D5173 − 97(Reapproved 2007)
Standard Test Method for
On-Line Monitoring of Carbon Compounds in Water by
Chemical Oxidation, by UV Light Oxidation, by Both, or by
High Temperature Combustion Followed by Gas Phase
NDIR or by Electrolytic Conductivity
This standard is issued under the fixed designation D5173; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D2777 Practice for Determination of Precision and Bias of
Applicable Test Methods of Committee D19 on Water
1.1 This test method covers the selection, establishment,
D3370 Practices for Sampling Water from Closed Conduits
and application of monitoring systems for carbon and carbon
D3694 Practices for Preparation of Sample Containers and
compounds by continual sampling or continuous flow-through,
for Preservation of Organic Constituents
automatic analysis, and recording or otherwise signaling of
D3864 Guide for On-Line Monitoring Systems for Water
output data. The system chosen will depend on the purpose for
Analysis
which it is intended (for example, regulatory compliance,
D4453 Practice for Handling of High Purity Water Samples
process monitoring, or to alert the user to adverse trends) and
D4779 Test Method for Total, Organic, and Inorganic Car-
on the type of water to be monitored (low purity or high purity,
bon in High Purity Water by Ultraviolet (UV) or Persul-
with or without suspended particulates, purgeable organics, or
fate Oxidation, or Both, and Infrared Detection (With-
inorganic carbon). If it is to be used for regulatory compliance,
drawn 2002)
the test method published or referenced in the regulations
D4839 Test Method forTotal Carbon and Organic Carbon in
should be used in conjunction with this test method and other
WaterbyUltraviolet,orPersulfateOxidation,orBoth,and
ASTM test methods. The test method covers carbon concen-
Infrared Detection
trations of 10 µg/L to 5000 mg/L.
3. Terminology
1.2 The values stated in SI units are to be regarded as the
standard.
3.1 Definitions—For definitions of terms used in this test
1.3 This standard does not purport to address all of the method, refer to Terminology D1129 and Guide D3864.
safety concerns, if any, associated with its use. It is the
4. Summary of Test Method
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- 4.1 A representative sample of a water stream, or the water
stream itself flows into a reaction chamber where all or some
bility of regulatory limitations prior to use. For specific hazard
statements, see Section 9. of the dissolved organic carbon is oxidized to carbon dioxide
by either of two means: (1) a chemical oxidant, an energy
2. Referenced Documents source such as ultraviolet (UV) radiation, or both, or (2) high
2 temperature combustion. This carbon dioxide is subsequently
2.1 ASTM Standards:
measured in the gas phase by a non-dispersive infrared
D1129 Terminology Relating to Water
detector, or is measured in solution by means of electrolytic
D1193 Specification for Reagent Water
conductivity. Interference may occur from the latter method if
the water sample has a high conductivity.
4.2 If there are suspended solids in the water stream, it is
This test method is under the jurisdiction of ASTM Committee D19 on Water
and is the direct responsibility of Subcommittee D19.03 on Sampling Water and
advisable to filter them out to prevent accumulation and
Water-Formed Deposits, Analysis of Water for Power Generation and Process Use,
possible blockage in the analyzer. The instrument will then
On-Line Water Analysis, and Surveillance of Water.
measure dissolved carbon plus any particulate carbon that
Current edition approved June 15, 2007. Published July 2007. Originally
approved in 1991. Last previous edition approved in 2001 as D5173 – 97 (2001). passes the filter. This parameter is usually called dissolved
DOI: 10.1520/D5173-97R07.
carbon.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The last approved version of this historical standard is referenced on
the ASTM website. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5173 − 97 (2007)
4.3 If there is inorganic carbon present in the water (in the 7. Apparatus
form of carbonate, bicarbonate, or carbon dioxide), it will also
7.1 Figs. 1-4 show in block diagram form several designs of
be detected as carbon dioxide. If inorganic carbon is not
on-line total organic carbon (TOC) analyzers that have been
removed before analysis, the monitor will report total carbon.
successfully introduced.
4.4 Inorganic carbon is removed from the water stream by
8. Reagents and Materials
acidifying and sparging the sample. This process may also
remove purgeable organic compounds. 8.1 Purity of Reagents—Reagent grade chemicals shall be
usedinalltests.Unlessotherwiseindicated,allreagentsshould
4.5 Suspended elemental carbon will not be oxidized by
conform to the specifications of the Committee on Analytical
low-temperature methods.
Reagents of the American Chemical Society. Other grades
may be used, provided it is first ascertained that the reagent is
5. Significance and Use
of sufficient purity to permit its use without decreasing the
5.1 Accuratemeasurementoforganiccarboninwateratlow
accuracy of the determinations.
and very low levels is of particular interest to the electronic,
8.2 Purity of Water:
pharmaceutical, and steam power generation industries.
8.2.1 Unless otherwise stated, references to reagent water
5.2 Elevated levels of organics in raw water tend to degrade
shall be understood to mean that conforming to Specification
ionexchangeresincapacity.Elevatedlevelsoforganicsinhigh D1193, Type II. The carbon content of this water should be
purity water tend to support biological growth and, in some
measured regularly by a suitably sensitive test method, such as
cases,aredirectlydetrimentaltotheprocessesthatrequirehigh Test Method D4779. It will typically be less than 0.2 mg/L
purity water.
carbon.
8.2.2 Water as free as possible of organics is desirable when
5.3 In the case of steam power generation, naturally occur-
establishing the test method blank at carbon levels of less than
ring organics can become degraded to CO and low molecular
1 mg/L. Absolutely carbon-free water is not obtainable in
weight organic acids that, in turn, are corrosive to the process
ordinary circumstances. However, a working approximation to
equipment. Their effect on conductivity may also cause water
this goal is the solution contained in the reaction vessel of
chemistry operating parameters to be exceeded, calling for
plant shutdown.
5.4 In process water in other industries, organic carbon can
Reagent Chemicals, American Chemical Society Specifications , American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
signify in-leakage of substances through damaged piping and
listed by the American Chemical Society, see Analar Standards for Laboratory
components, or an unacceptable level of product loss.
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
5.5 In wastewater treatment, organic carbon measurement
MD.
of influent and in-process water can help adjust optimize
treatment schemes. Measurement of organic carbon at dis-
charge may contribute to regulatory compliance.
6. Interferences
6.1 If inorganic carbon (dissolved CO and ions in equilib-
rium with it) is present, it will give a false positive to an
organic carbon measurement. Ion exchange resins used for
high purity water production typically strip CO from the
water, so this interferent is absent from such water unless the
water stream comes in contact with the atmosphere prior to
analysis.
6.2 If electrolytic conductivity is used for the measurement
of CO , other conductive species in solution will cause a
positive interference unless their background conductivity is
measured and deducted.
6.3 Particulates suspended in the water stream may cause
blockage in the monitor over a period of time, and may also be
hard to oxidize. If problems are anticipated, the water stream
NOTE 1—The unit employs available water system pressure to rinse the
should be appropriately filtered upstream of the monitor. The
line and test chamber, followed by a downstream valve closure that
parameter measured in the filtered water will be dissolved
isolates the sample. Subsequent irradiation with intense UV light breaks
organic carbon (DOC). down organic compounds in the water, with the liberated carbon forming
carbon dioxide in solution as carbonic acid. By monitoring the change in
6.4 Non-dispersive infrared detectors tuned to CO absor-
sample conductivity, corrected for temperature, the TOC concentration is
bance are also sensitive to water vapor, which may therefore
calculated and displayed.
give a positive interference unless removed. FIG. 1 Low Temperature Unit
D5173 − 97 (2007)
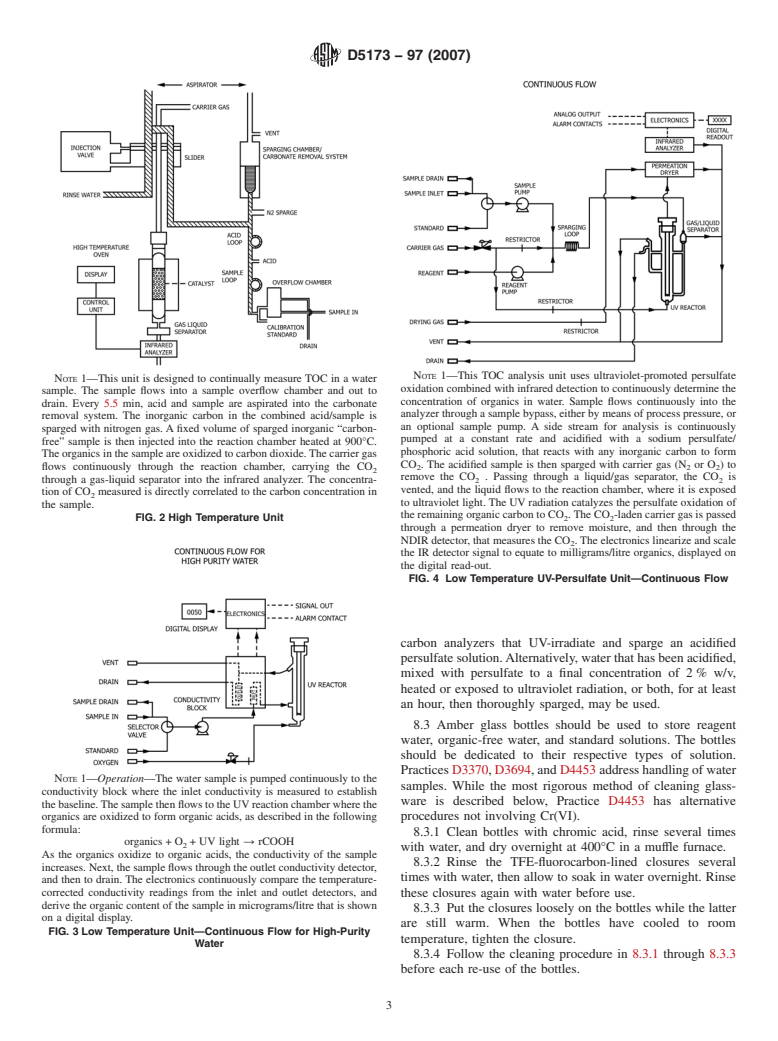
NOTE 1—This TOC analysis unit uses ultraviolet-promoted persulfate
NOTE 1—This unit is designed to continually measure TOC in a water
oxidation combined with infrared detection to continuously determine the
sample. The sample flows into a sample overflow chamber and out to
concentration of organics in water. Sample flows continuously into the
drain. Every 5.5 min, acid and sample are aspirated into the carbonate
analyzer through a sample bypass, either by means of process pressure, or
removal system. The inorganic carbon in the combined acid/sample is
an optional sample pump. A side stream for analysis is continuously
sparged with nitrogen gas. A fixed volume of sparged inorganic “carbon-
pumped at a constant rate and acidified with a sodium persulfate/
free” sample is then injected into the reaction chamber heated at 900°C.
phosphoric acid solution, that reacts with any inorganic carbon to form
The organics in the sample are oxidized to carbon dioxide.The carrier gas
CO . The acidified sample is then sparged with carrier gas (N or O)to
flows continuously through the reaction chamber, carrying the CO
2 2 2
remove the CO . Passing through a liquid/gas separator, the CO is
through a gas-liquid separator into the infrared analyzer. The concentra- 2 2
vented, and the liquid flows to the reaction chamber, where it is exposed
tion of CO measured is directly correlated to the carbon concentration in
to ultraviolet light. The UV radiation catalyzes the persulfate oxidation of
the sample.
the remaining organic carbon to CO .The CO -laden carrier gas is passed
FIG. 2 High Temperature Unit 2 2
through a permeation dryer to remove moisture, and then through the
NDIR detector, that measures the CO .The electronics linearize and scale
the IR detector signal to equate to milligrams/litre organics, displayed on
the digital read-out.
FIG. 4 Low Temperature UV-Persulfate Unit—Continuous Flow
carbon analyzers that UV-irradiate and sparge an acidified
persulfate solution.Alternatively, water that has been acidified,
mixed with persulfate to a final concentration of 2 % w/v,
heated or exposed to ultraviolet radiation, or both, for at least
an hour, then thoroughly sparged, may be used.
8.3 Amber glass bottles should be used to store reagent
water, organic-free water, and standard solutions. The bottles
should be dedicated to their respective types of solution.
Practices D3370, D3694, and D4453 address handling of water
NOTE 1—Operation—The water sample is pumped continuously to the
samples. While the most rigorous method of cleaning glass-
conductivity block where the inlet conductivity is measured to establish
ware is described below, Practice D4453 has alternative
the baseline.The sample then flows to the UVreaction chamber where the
organics are oxidized to form organic acids, as described in the following procedures not involving Cr(VI).
formula:
8.3.1 Clean bottles with chromic acid, rinse several times
organics + O + UV light→ rCOOH
with water, and dry overnight at 400°C in a muffle furnace.
As the organics oxidize to organic acids, the conductivity of the sample
8.3.2 Rinse the TFE-fluorocarbon-lined closures several
increases. Next, the sample flows through the outlet conductivity detector,
times with water, then allow to soak in water overnight. Rinse
and then to drain. The electronics continuously compare the temperature-
corrected conductivity readings from the inlet and outlet detectors, and
these closures again with water before use.
derive the organic content of the sample in micrograms/litre that is shown
8.3.3 Put the closures loosely on the bottles while the latter
on a digital display.
are still warm. When the bottles have cooled to room
FIG. 3 Low Temperature Unit—Continuous Flow for High-Purity
temperature, tighten the closure.
Water
8.3.4 Follow the cleaning procedure in 8.3.1 through 8.3.3
before each re-use of the bottles.
D5173 − 97 (2007)
8.4 Gas Supply—Use a gas free of CO and organic matter, of sophistication and complexity, depending on the specific
ofapurityasspecifiedbytheequipmentmanufacturer.Oxygen nature of the application.
is recommended.
10.5 Review all sample requirements with the equipment
8.5 Organic Carbon Solution, Standard: supplier. Be sure to define accurately all conditions of intended
8.5.1 Prepare high-concentration calibration standards operation, the components in the sample and expected varia-
(2000 mg/L carbon) using a water-soluble, stable compound. tions in the measured parameters.
This stock solution can then be further diluted to a concentra-
10.6 Materials of construction that will be in contact with
tion suitable for the method used. (See 8.5 of Test Method
the sample should be such that will not react with the sample.
D4779.)
Note that high-purity water monitoring may demand a mini-
8.5.2 The compound used for calibration should be as
mum of organic polymers in the monitor, while certain process
similar as possible to the compound(s) expected to be present
and waste streams may be highly corrosive and may therefore
in the water to be analyzed.
require inert polymers to be used.
9. Hazards
10.7 Select the sampling point(s) so as to provide a repre-
sentative and measu
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.