ASTM F3259-17
(Guide)Standard Guide for Micro-computed Tomography of Tissue Engineered Scaffolds
Standard Guide for Micro-computed Tomography of Tissue Engineered Scaffolds
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F3259 − 17
Standard Guide for
Micro-computed Tomography of Tissue Engineered
1
Scaffolds
This standard is issued under the fixed designation F3259; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.6 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
1.1 Thisguideisaresourceforconductingmicro-computed
responsibility of the user of this standard to establish appro-
tomography (microCT) imaging and analysis of porous scaf-
priate safety and health practices and determine the applica-
folds for tissue engineering applications. Considerations are
bility of regulatory limitations prior to use.
provided for sample preparation, image acquisition parameter
1.7 This international standard was developed in accor-
selection, post-processing, and data interpretation.
dance with internationally recognized principles on standard-
1.2 The information in this guide is intended to be appli-
ization established in the Decision on Principles for the
cabletoproductsthatincludeaporousscaffoldcomponentand
Development of International Standards, Guides and Recom-
are designed for tissue engineering repair strategies. The
mendations issued by the World Trade Organization Technical
scaffolds may be fabricated from synthetic polymers (e.g.,
Barriers to Trade (TBT) Committee.
absorbable polyesters) or natural materials (e.g., calcium
phosphates), mammalian or human derived materials (e.g.,
2. Referenced Documents
demineralized bone) or combinations of these. While some
3
2.1 ASTM Standards:
considerationsareprovidedforimagingofmaterialsthatareof
F2450Guide for Assessing Microstructure of Polymeric
moderate to high radiodensity, specific guidelines are not
Scaffolds for Use in Tissue-Engineered Medical Products
provided for imaging metallic scaffolds.
F2603Guide for Interpreting Images of Polymeric Tissue
1.3 Applicability of the guidelines herein will depend on
Scaffolds
scaffold material type and the user’s application (e.g., experi-
mental design, as manufactured characterization) as appropri-
3. Terminology
ate.
3.1 Definitions of Terms Specific to This Standard:
1.4 The guidelines for microCT discussed herein are most
3.1.1 microarchitecture, n—the set of structural features of
suitable for specimen scanning in vitro. Specific guidelines
an object defined at the microscale.
relevant to direct in vivo imaging of scaffolds are not included
3.1.2 volume of interest (VOI), n—a 3D sub-volume inside
because the imaging parameters will be dependent on the
an image that contains the features to be analyzed.
implantation site, animal size, breathing etc. In addition,
consensus recommendations for in vivo imaging are provided
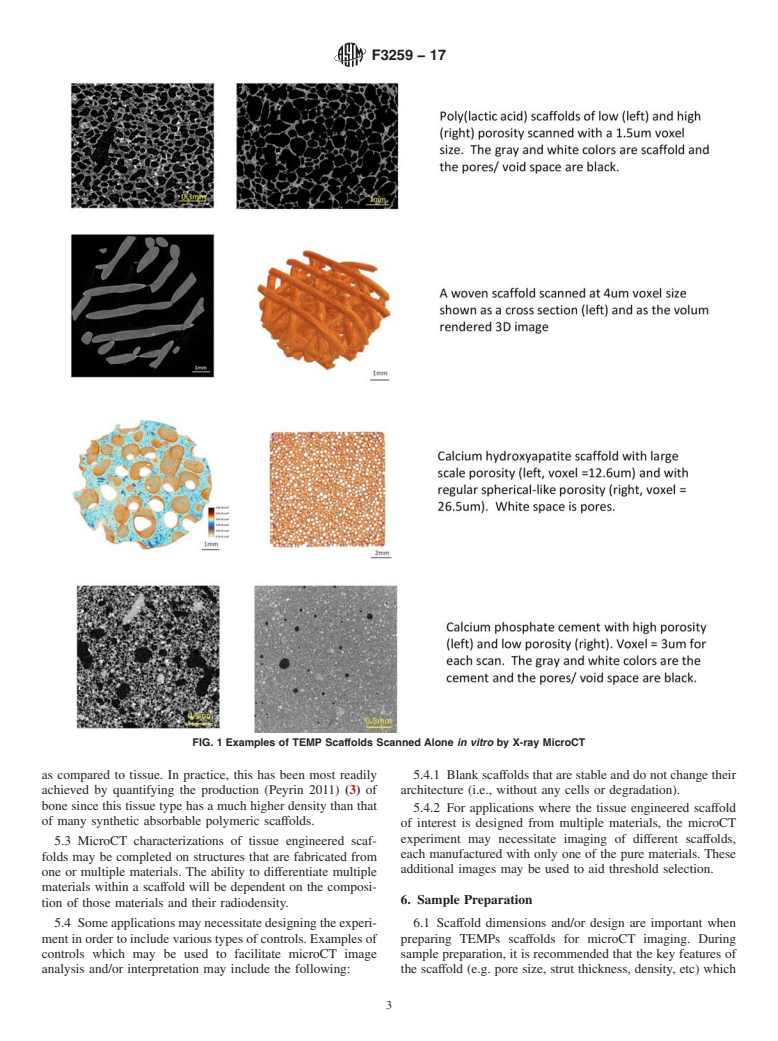
4. Significance and Use
2
in Bouxsein et al 2010 (1). While the specific imaging
4.1 X-ray microcomputed tomography (microCT) is a non-
parameters and processing recommendations discussed in
destructivethree-dimensionalimagingmethodthatcanbeused
Bouxsein et al are specific to bone imaging, many of the
to reconstruct the microarchitecture of a tissue engineered
considerations and precautions are also applicable for in vivo
medical product (TEMP) scaffold that may or may not contain
scaffold imaging.
ingrowntissue.MicroCTwasfirstdevelopedtostudyceramics
1.5 The values stated in SI units are to be regarded as
for the auto-industry and adapted for bone morphology at the
standard. No other units of measurement are included in this
microscale (Feldkamp et al 1989) (2). More recently, the
standard.
imaging method has been adapted for in vivo applications and
studies of multiple natural and synthetic materials.
1
This guide is under the jurisdiction ofASTM Committee F04 on Medical and
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.42 on Biomaterials and Biomolecules for TEMPs.
3
Current edition approved May 1, 2017. Published September 2017. DOI: For referenced ASTM standards, visit the ASTM website, www.astm.org, or
10.1520/F3259-17. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
2
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof Standards volume information, refer to the standard’s Document Summary page on
this standard. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 --------------------
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.