ASTM D5128-90(1999)e1
(Test Method)Standard Test Method for On-Line pH Measurement of Water of Low Conductivity
Standard Test Method for On-Line pH Measurement of Water of Low Conductivity
SCOPE
1.1 This test method covers the precise on-line determination of pH in water samples of conductivity lower than 100 [mu]S/cm (see Tables 1 and 2) over the pH range of 3 to 11 (see Fig. 1), under field operating conditions, utilizing a sealed, non-refillable, reference electrode. pH measurements of water of low conductivity are problematical for conventional pH electrodes, methods, and related measurement apparatus.
1.2 This test method includes the procedures and equipment required for the continuous pH measurement of low conductivity water sample streams including the requirements for the control of sample stream pressure, flow rate, and temperature.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
e1
Designation: D 5128 – 90 (Reapproved 1999)
Standard Test Method for
On-Line pH Measurement of Water of Low Conductivity
This standard is issued under the fixed designation D5128; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—A footnote was editorially removed in June 1999.
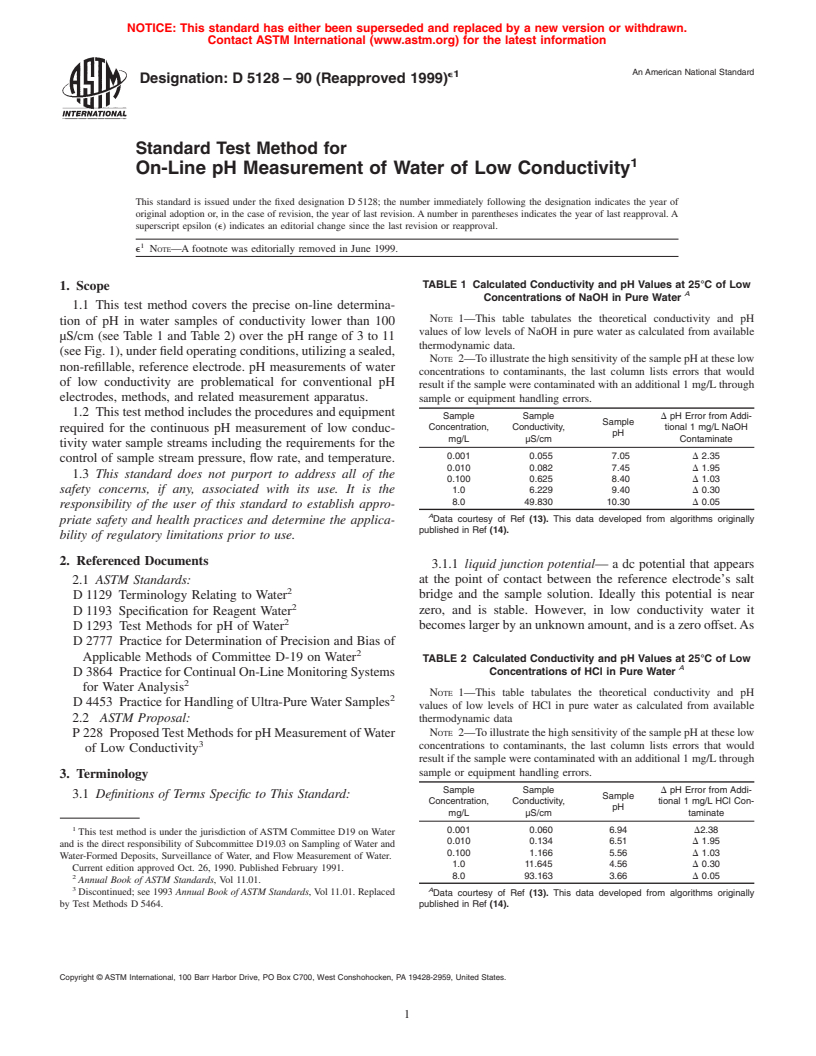
TABLE 1 Calculated Conductivity and pH Values at 25°C of Low
1. Scope
A
Concentrations of NaOH in Pure Water
1.1 This test method covers the precise on-line determina-
NOTE 1—This table tabulates the theoretical conductivity and pH
tion of pH in water samples of conductivity lower than 100
values of low levels of NaOH in pure water as calculated from available
µS/cm (see Table 1 and Table 2) over the pH range of 3 to 11
thermodynamic data.
(seeFig.1),underfieldoperatingconditions,utilizingasealed,
NOTE 2—ToillustratethehighsensitivityofthesamplepHattheselow
non-refillable, reference electrode. pH measurements of water
concentrations to contaminants, the last column lists errors that would
of low conductivity are problematical for conventional pH
result if the sample were contaminated with an additional 1 mg/Lthrough
electrodes, methods, and related measurement apparatus.
sample or equipment handling errors.
1.2 Thistestmethodincludestheproceduresandequipment
Sample Sample D pH Error from Addi-
Sample
Concentration, Conductivity, tional 1 mg/L NaOH
required for the continuous pH measurement of low conduc-
pH
mg/L µS/cm Contaminate
tivity water sample streams including the requirements for the
0.001 0.055 7.05 D 2.35
control of sample stream pressure, flow rate, and temperature.
0.010 0.082 7.45 D 1.95
1.3 This standard does not purport to address all of the
0.100 0.625 8.40 D 1.03
safety concerns, if any, associated with its use. It is the
1.0 6.229 9.40 D 0.30
8.0 49.830 10.30 D 0.05
responsibility of the user of this standard to establish appro-
A
Data courtesy of Ref (13). This data developed from algorithms originally
priate safety and health practices and determine the applica-
published in Ref (14).
bility of regulatory limitations prior to use.
2. Referenced Documents
3.1.1 liquid junction potential— a dc potential that appears
at the point of contact between the reference electrode’s salt
2.1 ASTM Standards:
bridge and the sample solution. Ideally this potential is near
D1129 Terminology Relating to Water
D1193 Specification for Reagent Water zero, and is stable. However, in low conductivity water it
becomeslargerbyanunknownamount,andisazerooffset.As
D1293 Test Methods for pH of Water
D2777 Practice for Determination of Precision and Bias of
Applicable Methods of Committee D-19 on Water
TABLE 2 Calculated Conductivity and pH Values at 25°C of Low
A
Concentrations of HCl in Pure Water
D3864 PracticeforContinualOn-LineMonitoringSystems
for Water Analysis
NOTE 1—This table tabulates the theoretical conductivity and pH
D4453 PracticeforHandlingofUltra-PureWaterSamples
values of low levels of HCl in pure water as calculated from available
2.2 ASTM Proposal: thermodynamic data
NOTE 2—ToillustratethehighsensitivityofthesamplepHattheselow
P228 ProposedTestMethodsforpHMeasurementofWater
concentrations to contaminants, the last column lists errors that would
of Low Conductivity
result if the sample were contaminated with an additional 1 mg/Lthrough
sample or equipment handling errors.
3. Terminology
Sample Sample D pH Error from Addi-
3.1 Definitions of Terms Specific to This Standard: Sample
Concentration, Conductivity, tional 1 mg/L HCl Con-
pH
mg/L µS/cm taminate
0.001 0.060 6.94 D2.38
This test method is under the jurisdiction ofASTM Committee D19 on Water
0.010 0.134 6.51 D 1.95
and is the direct responsibility of Subcommittee D19.03 on Sampling of Water and
0.100 1.166 5.56 D 1.03
Water-Formed Deposits, Surveillance of Water, and Flow Measurement of Water.
1.0 11.645 4.56 D 0.30
Current edition approved Oct. 26, 1990. Published February 1991.
8.0 93.163 3.66 D 0.05
Annual Book of ASTM Standards, Vol 11.01.
3 A
Discontinued; see 1993 Annual Book of ASTM Standards, Vol 11.01. Replaced Data courtesy of Ref (13). This data developed from algorithms originally
by Test Methods D5464. published in Ref (14).
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
e1
D 5128 – 90 (1999)
4.2 Thistestmethoddescribestheapparatusandprocedures
to be used for the continuous on-line pH measurement of low
conductivity water sample streams. The type of pH sensor
assembly and pH instrument interface module are described in
detail. The requirements for sample stream manifolds for the
conditioning of sample pressure and flow rate are defined, and
arrangements for this associated equipment are illustrated.
Guidelines for the proper installation and calibration of the pH
sensor and associated sample manifold are discussed along
with the precautions that must be considered concerning
sample contamination and representative sampling for calibra-
tion purposes.
4.3 The apparatus and procedures described in this test
method are intended to be used with most state-of-the-art,
process-grade,pHanalyzer/transmitterinstrumentscurrentlyin
use or available from the major manufacturers of such instru-
mentation.
5. Significance and Use
5.1 pH measurements are typically made in solutions that
contain relatively large amounts of acid, base, or dissolved
salts. Under these conditions, pH determinations may be made
quicklyandprecisely.Continuouson-linepHmeasurementsin
water samples of low conductivity are more difficult (4, 5).
These low ionic strength solutions are susceptible to contami-
nation from the atmosphere, sample stream hardware, and the
pH electrodes. Variations in the constituent concentration of
low conductivity waters cause liquid junction potential shifts
(see 3.1.1) resulting in pH measurement errors.The aggressive
FIG. 1 Restrictions Imposed by the Conductivity pH Relationship
nature and the high electrical resistance of pure and ultra-pure,
low conductivity waters may degrade the pH measurement
long as it remains stable its effect can be minimized by “grab
electrodes resulting in unstable and drifting pH output signals.
sample” calibration (1).
5.2 It is essential to make on-line pH measurements of low
3.1.2 streaming potential—thestaticelectricalchargethatis
conductivity water as accurately as possible to determine the
induced by the movement of a low ionic strength solution
propercontrolofpHadjustmentchemicals,theeffectivenessof
having a high electrical resistivity or low electrical conductiv-
demineralizer equipment, the event and nature of impurity
ity (such as pure water), across relatively non-conductive
contamination of the water, and information pertaining to the
surfaces such as the pH measurement electrode’s glass mem-
overall status of the pure water system.
brane or other non-conductive wetted materials found in
flowing sample streams.
6. Interferences
3.2 Definitions: Definitions—For definitions of other terms
6.1 Samplesystemsforhighpurity,lowconductivitywaters
used in this test method, refer to Terminology D1129 and
are especially sensitive to contamination from atmospheric
Practice D3864.
gases (especially carbon dioxide, see Appendix X1 and Table
3) from collection of “crud” (insoluble deposits of iron oxide
4. Summary of Test Method
and other by-products of metallic corrosion that are present
4.1 pH is measured by a pair of electrodes contained in an
throughout the system) in sample lines, from exposure to high
all stainless steel flow cell. The pH measurement half cell is
constructed of a glass membrane suitable for continuous
TABLE 3 Calculated pH and Conductivity Values at 25°C of
serviceinlowconductivitywater.ManymodernpHelectrodes
A
Water Solutions Containing Only Ammonia and Carbon Dioxide
are available that perform well in this service. However, the
pH Shift
bulb impedance should be kept low to minimize the effects of“
Carbon Dioxide Carbon Dioxide
Caused by
0 mg/L 0.2 mg/L
streaming potential” (see 3.1.2). The reference half cell is
Ammonia 0.2 mg/L
mg/L CO
sealed (requiring no electrolyte replenishment) and is con-
Contamination
µS/cm pH µS/cm pH
structed in such a manner that the salt bridge, while making
of Sample
diffusion contact to the sample, resists significant dilution for
0 0.056 7.00 0.508 5.89 D 1.11 pH
periods up to several months on continuous operation.
0.12 1.462 8.73 1.006 8.18 D 0.55 pH
0.51 4.308 9.20 4.014 9.09 D 0.11 pH
0.85 6.036 9.34 5.788 9.26 D 0.08 pH
1.19 7.467 9.44 7.246 9.38 D 0.06 pH
The boldface numbers given in parentheses refer to a list of references at the
A
end of this standard. Data extracted from Ref (15).
e1
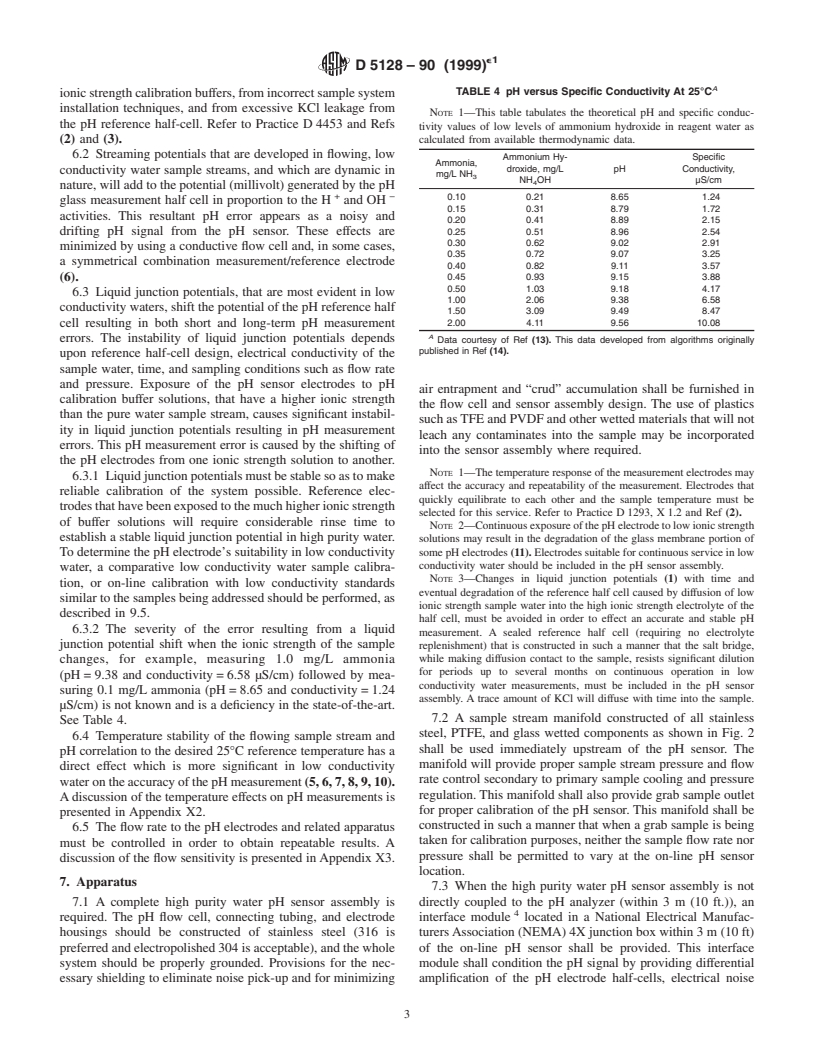
D 5128 – 90 (1999)
A
TABLE 4 pH versus Specific Conductivity At 25°C
ionicstrengthcalibrationbuffers,fromincorrectsamplesystem
installation techniques, and from excessive KCl leakage from
NOTE 1—This table tabulates the theoretical pH and specific conduc-
the pH reference half-cell. Refer to Practice D4453 and Refs
tivity values of low levels of ammonium hydroxide in reagent water as
(2) and (3). calculated from available thermodynamic data.
6.2 Streaming potentials that are developed in flowing, low
Ammonium Hy- Specific
Ammonia,
droxide, mg/L pH Conductivity,
conductivity water sample streams, and which are dynamic in
mg/L NH
NH OH µS/cm
nature,willaddtothepotential(millivolt)generatedbythepH
+ −
0.10 0.21 8.65 1.24
glass measurement half cell in proportion to the H and OH
0.15 0.31 8.79 1.72
activities. This resultant pH error appears as a noisy and
0.20 0.41 8.89 2.15
0.25 0.51 8.96 2.54
drifting pH signal from the pH sensor. These effects are
0.30 0.62 9.02 2.91
minimized by using a conductive flow cell and, in some cases,
0.35 0.72 9.07 3.25
a symmetrical combination measurement/reference electrode
0.40 0.82 9.11 3.57
(6). 0.45 0.93 9.15 3.88
0.50 1.03 9.18 4.17
6.3 Liquid junction potentials, that are most evident in low
1.00 2.06 9.38 6.58
conductivitywaters,shiftthepotentialofthepHreferencehalf
1.50 3.09 9.49 8.47
cell resulting in both short and long-term pH measurement 2.00 4.11 9.56 10.08
A
errors. The instability of liquid junction potentials depends
Data courtesy of Ref (13). This data developed from algorithms originally
published in Ref (14).
upon reference half-cell design, electrical conductivity of the
sample water, time, and sampling conditions such as flow rate
and pressure. Exposure of the pH sensor electrodes to pH
air entrapment and “crud” accumulation shall be furnished in
calibration buffer solutions, that have a higher ionic strength
the flow cell and sensor assembly design. The use of plastics
than the pure water sample stream, causes significant instabil-
suchasTFEandPVDFandotherwettedmaterialsthatwillnot
ity in liquid junction potentials resulting in pH measurement
leach any contaminates into the sample may be incorporated
errors. This pH measurement error is caused by the shifting of
into the sensor assembly where required.
the pH electrodes from one ionic strength solution to another.
NOTE 1—Thetemperatureresponseofthemeasurementelectrodesmay
6.3.1 Liquidjunctionpotentialsmustbestablesoastomake
affect the accuracy and repeatability of the measurement. Electrodes that
reliable calibration of the system possible. Reference elec-
quickly equilibrate to each other and the sample temperature must be
trodesthathavebeenexposedtothemuchhigherionicstrength
selected for this service. Refer to Practice D1293, X1.2 and Ref (2).
of buffer solutions will require considerable rinse time to
NOTE 2—ContinuousexposureofthepHelectrodetolowionicstrength
establish a stable liquid junction potential in high purity water.
solutions may result in the degradation of the glass membrane portion of
somepHelectrodes(11).Electrodessuitableforcontinuousserviceinlow
TodeterminethepHelectrode’ssuitabilityinlowconductivity
conductivity water should be included in the pH sensor assembly.
water, a comparative low conductivity water sample calibra-
NOTE 3—Changes in liquid junction potentials (1) with time and
tion, or on-line calibration with low conductivity standards
eventual degradation of the reference half cell caused by diffusion of low
similartothesamplesbeingaddressedshouldbeperformed,as
ionic strength sample water into the high ionic strength electrolyte of the
described in 9.5.
half cell, must be avoided in order to effect an accurate and stable pH
6.3.2 The severity of the error resulting from a liquid
measurement. A sealed reference half cell (requiring no electrolyte
junction potential shift when the ionic strength of the sample
replenishment) that is constructed in such a manner that the salt bridge,
while making diffusion contact to the sample, resists significant dilution
changes, for example, measuring 1.0 mg/L ammonia
for periods up to several months on continuous operation in low
(pH=9.38 and conductivity=6.58 µS/cm) followed by mea-
conductivity water measurements, must be included in the pH sensor
suring 0.1 mg/L ammonia (pH=8.65 and conductivity=1.24
assembly. A trace amount of KCl will diffuse with time into the sample.
µS/cm) is not known and is a deficiency in the state-of-the-art.
7.2 A sample stream manifold constructed of all stainless
See Table 4.
steel, PTFE, and glass wetted components as shown in Fig. 2
6.4 Temperature stability of the flowing sample stream and
shall be used immediately upstream of the pH sensor. The
pH correlation to the desired 25°C reference temperature has a
manifold will provide proper sample stream pressure and flow
direct effect which is more significant in low conductivity
rate control secondary to primary sample cooling and pressure
waterontheaccuracyofthepHmeasurement(5,6,7,8,9,10).
regulation.This manifold shall also provide grab sample outlet
AdiscussionofthetemperatureeffectsonpHmeasurementsis
for proper calibration of the pH sensor. This manifold shall be
presented in Appendix X2.
constructed in such a manner that when a grab sample is being
6.5 The flow rate to the pH electrodes and related apparatus
taken for calibration purposes, neither the sample flow rate nor
must be controlled in order to obtain repeatable results. A
pressure shall be permitted to vary at the on-line pH sensor
discussion of the flow sensitivity is presented inAppendix X3.
location.
7. Apparatus
7.3 When the high purity water pH sensor assembly is not
7.1 A complete high purity water pH sensor assembly is directly coupled to the pH anal
...






Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.