ASTM E1343-90(1997)e1
(Test Method)Standard Test Method for Molecular Weight Cutoff Evaluation of Flat Sheet Ultrafiltration Membranes
Standard Test Method for Molecular Weight Cutoff Evaluation of Flat Sheet Ultrafiltration Membranes
SCOPE
1.1 This test method covers the evaluation of the molecular weight cutoff of flat sheet ultrafiltration membranes with cutoffs between 4500 and 1000000 daltons. The nonadsorbing characteristics of the test penetrant utilized by this test method permit the test to be performed on a wide variety of membrane substrates, excluding those which strongly adsorb dextran, from highly hydrophilic to highly hydrophobic. This test method is not applicable for microfiltration membranes with pore sizes of 0.01 µm or larger, nor for reverse osmosis or dialysis membranes with less than 4500 molecular weight cutoff. (It is possible that this test method could be modified to expand the range from 100 to 2 000 000 daltons.) This test method is not applicable to membrane materials that strongly adsorb dextrans since these materials will potentially change the value of the measured molecular weight cutoff and hence will invalidate the test results.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
e1
Designation: E 1343 – 90 (Reapproved 1997)
Standard Test Method for
Molecular Weight Cutoff Evaluation of Flat Sheet
Ultrafiltration Membranes
This standard is issued under the fixed designation E 1343; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—Section 11 was added editorially in April 1997.
1. Scope tration membranes. The use of nonfouling dextrans allows a
direct comparison of membranes without interference from
1.1 This test method covers the evaluation of the molecular
materials that may foul one membrane and not affect another.
weight cutoff of flat sheet ultrafiltration membranes with
The degree of correlation between this test and actual perfor-
cutoffs between 4500 and 1 000 000 daltons. The nonadsorbing
mance on a commercial feed stream has not been completely
characteristics of the test penetrant utilized by this test method
established; however, a membrane can be exposed to the
permit the test to be performed on a wide variety of membrane
fouling solution in question and then the effect of that foulant
substrates, excluding those which strongly adsorb dextran,
determined by then running the test and comparing to the
from highly hydrophilic to highly hydrophobic. This test
results on an appropriate unfouled membrane. It should be
method is not applicable for microfiltration membranes with
made clear that this test method does not substitute for the
pore sizes of 0.01 μm or larger, nor for reverse osmosis or
actual testing of a commercial or experimental membrane on a
dialysis membranes with less than 4500 molecular weight
feed stream of interest. The low transmembrane pressures, lack
cutoff. (It is possible that this test method could be modified to
of adsorption of the test permeants onto the membrane, and
expand the range from 100 to 2 000 000 daltons.) This test
low recovery/pass are intended to eliminate interferences such
method is not applicable to membrane materials that strongly
as polarization and fouling that mask the properties of the
adsorb dextrans since these materials will potentially change
membrane. It is likely that any system operated in a commer-
the value of the measured molecular weight cutoff and hence
cial fashion will experience fouling, adsorption, and polariza-
will invalidate the test results.
tion to some degree as well as a “compaction” phenomenon
1.2 This standard does not purport to address all of the
over the first several h of operation.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
4. Apparatus
priate safety and health practices and determine the applica-
4.1 The basic membrane test system consists of the follow-
bility of regulatory limitations prior to use.
ing:
2. Summary of Test Method 4.1.1 Standard Flat Membrane, Stirred Ultrafiltration Test
Cell, for 62-mm membrane disc, modified as shown in Fig. 1,
2.1 The membrane is rinsed with purified water and in-
4.1.2 Diaphragm Pump, approximately 0 to 200 mL/min
stalled in the test cell. The precalibrated dextran T-fraction feed
variable pumping range, 30 psig pressure capability at 100
is pumped through the cell and the flux through the membrane
mL/min, all wetted parts of 316 stainless steel and polyfluo-
is set at the value of 0.0001 cm/s and an ultrafiltrate sample
rocarbon construction,
taken and compared to a sample of the feed for molecular
4.1.3 Back Pressure Regulator and Pressure Gage,0to30
weight distribution. Gel permeation chromatography is used to
psig, 316 stainless steel,
compare the feed to the permeate and the rejection at each 3-s
4.1.4 Electronic Digital Flowmeter, with direct readout of
data slice is calculated. The resulting rejection is then plotted as
volumetric flow in the approximate range 0.06 to 5 mL/min,
a function of the molecular mass average of the sample for the
4.1.5 Magnetic Stirplate with Tachometer,
data slice.
3. Significance and Use
Membrane equipment, model 8200 or equal, available from Amicon Division
3.1 This test method provides a convenient, rapid, reproduc-
of W. R. Grace, 1114-T Avenue of the Americas, New York, NY 10036, has been
found suitable for this purpose.
ible method of comparing the intrinsic properties of ultrafil-
Diaphram pump, model EP-C40 or equal, available from CHEM/TECH
International Industries, 1655-T Des Peres P.O. Box 31000, St. Louis, MO 63131,
This test method is under the jurisdiction of ASTM Committee E-48 on has been found suitable for this purpose.
Biotechnology and is the direct responsibility of Subcommittee E48.03 on Unit Stirplate, model 4650-54, or its equivalent, available from Cole Parmer
Processes and Their Control. Spincadet, 7427-T N. Oak Park Ave., Chicago, IL 60648, has been found suitable for
Current edition approved March 30, 1990. Published May 1990. this purpose.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
E 1343
NOTE 1—The top of a standard stirred ultrafiltration cell was modified by drilling a hole through it and tapping it for ⁄8-in. male pipe thread (MPT).
1 1 1
A nylon fitting ( ⁄8-in. tube to ⁄8-in. MPT) was drilled through to allow the insertion of ⁄8-in. tubing. The fitting was threaded into the top of the test cell.
A stainless steel inlet tube was prepared as shown. The nylon fitting allows for the direction of flow to be at any height, and direction, or both. The existing
fitting in the cap is used as an exit of flow. If the cell is equipped with a wrap-around clamp, it is modified to accommodate the extra fitting.
FIG. 1 Flow Through Modification of an Amicon-Stirred Cell
4.1.6 Magnetic Stir Plate, Stirbar, and Container, (1000 cm 4.2.5 Differential Refractometer HPLC Detector, with 30°C
Erlenmeyer) for test solution, water recirculating in the detector block and associated ther-
4.1.7 Pulse Dampener, 500-Ml, 316 stainless steel, mostatically controlled water source 60.1°C,
4.1.8 Suitable Thermostatically Controlled Bath, capable of 4.2.6 Computing Integrator, with data slicing and BASIC
30 6 0.2°C, programming capabilities and suitable connecting cables for
4.1.9 Highly Reproducible and Stable Peristaltic Pump, the integrator and detector. The integrator should include a
capable of flows in the 0.06 to 5 mL/min range, printer capability to provide on-line output.
4.1.10 Appropriate Silicone Rubber Tubing, for the peristal-
5. Reagents and Materials
tic pump, and
4.1.11 Inline Filters, two, 2 and 15-μm, 316 stainless steel
5.1 Dextran T-fractions—10 000 (T-10); 40 000 (T-40);
construction. 70 000 (T-70); and 500 000 (T-500) average molecular mass,
4.2 The gel chromatography system consists of the follow-
supplied by the manufacturer with calibration curves of instan-
ing: taneous and cumulative distribution versus molecular mass.
4.2.1 Good Quality High-Performance Liquid Chromatog-
5.2 Purified Water—Treated with reverse osmosis, ion ex-
5 11
raphy (HPLC) Pump with Highly Reproducible Flow, change and 0.2-μm filter to 18 MV quality.
4.2.2 HPLC Injection Valve, with 20-mL sample loop and a
5.3 Sodium Azide.
position sensing switch,
6. Hazards
4.2.3 Pair of Gel Permeation Columns, capable of size
exclusion separation of dextran polysaccharides in the 4500 to 6.1 Warning—Sodium azide is toxic and flammable with a
6 7
10 molecular mass range,
tolerance of 1 ppm in air, while the dextran T-fractions are
4.2.4 Precolumn Filter to Protect the Columns (Guard nontoxic, the pressures utilized are hydraulic and are typically
Column),
less than one atmosphere.
7. Preparation of Apparatus
HPLC pump such as Beckman 110B Solvent Delivery Module, or its
7.1 Assembly of Equipment—Chromatograph:
equivalent, available from Beckman Instruments, Inc., 2500-T Harbor Blvd., L-19
Fullerton, CA, has been found suitable for this purpose.
Injection valve, such as 7010 HPLC, or its equivalent, available from Rheodyne
Inc., 6815-T S. Santa Rosa Ave., P.O. Box 996, Cotati, CA 94928 has been found Refractometer such as a R401 Differential, or its equivalent, available from
suitable for this purpose. Walters Inc., has been found suitable for this purpose.
7 10
Gel permeation columns such as Toyo-Soda G4000PW and G2000PW, with Computing integrator such as model SP.4270, or its equivalent, available from
matching guard column or equivalents, have been found suitable for this purpose. Spectra Physics, 5475-T Kellenberger Dr., Dayton, OH 45425, has been found
Filters such as #84 560, or equivalents, available from Walters Inc., Mausner suitable for this purpose.
Equipment Co. Inc., 1304-T Prospect Ave. East Meadows, NY, have been found System such as a Millipore Milli-Q, or its equivalent, available from Millipore
suitable for this purpose. Inc., 80 Ashby Rd., Bedford, MA, has been found suitable for this purpose.
E 1343
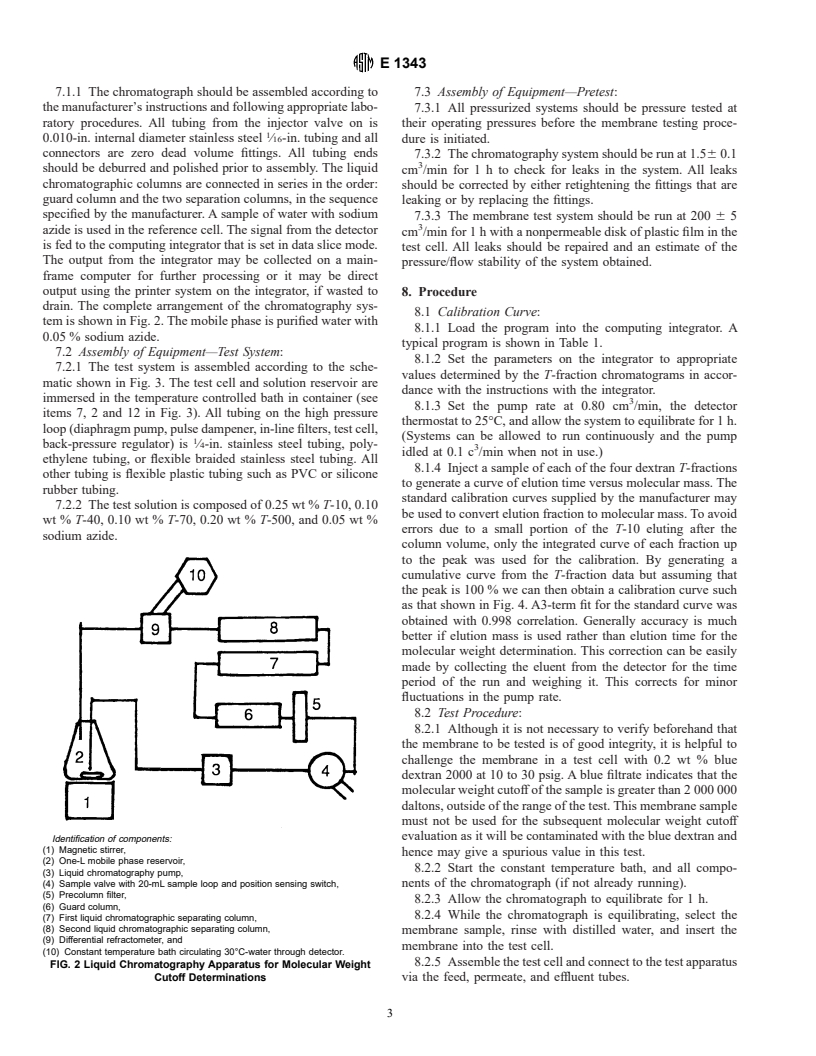
7.1.1 The chromatograph should be assembled according to 7.3 Assembly of Equipment—Pretest:
the manufacturer’s instructions and following appropriate labo- 7.3.1 All pressurized systems should be pressure tested at
ratory procedures. All tubing from the injector valve on is
their operating pressures before the membrane testing proce-
0.010-in. internal diameter stainless steel ⁄16-in. tubing and all dure is initiated.
connectors are zero dead volume fittings. All tubing ends
7.3.2 The chromatography system should be run at 1.56 0.1
should be deburred and polished prior to assembly. The liquid
cm /min for1hto check for leaks in the system. All leaks
chromatographic columns are connected in series in the order:
should be corrected by either retightening the fittings that are
guard column and the two separation columns, in the sequence
leaking or by replacing the fittings.
specified by the manufacturer. A sample of water with sodium
7.3.3 The membrane test system should be run at 200 6 5
azide is used in the reference cell. The signal from the detector
cm /min for 1 h with a nonpermeable disk of plastic film in the
is fed to the computing integrator that is set in data slice mode.
test cell. All leaks should be repaired and an estimate of the
The output from the integrator may be collected on a main-
pre
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.