ASTM D2779-92(2007)
(Test Method)Standard Test Method for Estimation of Solubility of Gases in Petroleum Liquids
Standard Test Method for Estimation of Solubility of Gases in Petroleum Liquids
SIGNIFICANCE AND USE
Knowledge of gas solubility is of extreme importance in the lubrication of gas compressors. It is believed to be a substantial factor in boundary lubrication, where the sudden release of dissolved gas may cause cavitation erosion, or even collapse of the fluid film. In hydraulic and seal oils, gas dissolved at high pressure can cause excessive foaming on release of the pressure. In aviation oils and fuels, the difference in pressure between take-off and cruise altitude can cause foaming out of the storage vessels and interrupt flow to the pumps.
SCOPE
1.1 This test method covers the estimation of the equilibrium solubility of several common gases encountered in the aerospace industry in hydrocarbon liquids. These include petroleum fractions with densities in the range from 0.63 to 0.90 at 288 K (59F). The solubilities can be estimated over the temperature range 228 K (-50°F) to 423 K (302°F).
1.2 This test method is based on the Clausius-Clapeyron equation, Henry's law, and the perfect gas law, with empirically assigned constants for the variation with density and for each gas.
1.3 The values stated in SI units are to be regarded as the standard. The values in parentheses are for information only.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D2779–92(Reapproved 2007)
Standard Test Method for
Estimation of Solubility of Gases in Petroleum Liquids
This standard is issued under the fixed designation D2779; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.1.3 Henry’s law—the principle that the ratio of partial
pressure to mole fraction of gas in solution is a constant.
1.1 This test method covers the estimation of the equilib-
3.1.3.1 Discussion—In non-ideal systems the fugacity is
rium solubility of several common gases encountered in the
used to replace the pressure, but the systems within the scope
aerospace industry in hydrocarbon liquids. These include
of this test method can be considered ideal within the limits of
petroleum fractions with densities in the range from 0.63 to
the accuracy statement.
0.90at288K(59°F).Thesolubilitiescanbeestimatedoverthe
3.2 Symbols:
temperature range 228 K (−50°F) to 423 K (302°F).
1.2 This test method is based on the Clausius-Clapeyron
equation, Henry’s law, and the perfect gas law, with empiri-
d = density of the liquid at 288 K (59°F), kg/L,
cally assigned constants for the variation with density and for
T = specified temperature, K,
each gas.
L = Ostwald coefficient at 273 K for a liquid of
o
1.3 The values stated in SI units are to be regarded as the
d=0.85,
standard. The values in parentheses are for information only.
L = Ostwald coefficient at T for a liquid of
1.4 This standard does not purport to address all of the
d=0.85,
safety concerns, if any, associated with its use. It is the
L = Ostwald coefficient at T for a liquid of the
c
responsibility of the user of this standard to establish appro-
specified density,
priate safety and health practices and determine the applica- p = pressure of the gas, or mixed gases, MPa,
bility of regulatory limitations prior to use. p = vapor pressure of the liquid at the specified
v
temperature, MPa,
2. Referenced Documents
p,p . p = partial pressures of the gases in a mixture,
1 2 i
2.1 ASTM Standards: MPa,
G = solubility, mg/kg,
D1298 Test Method for Density, Relative Density (Specific
B = Bunsencoefficientatthespecified d, p,and T,
Gravity), or API Gravity of Crude Petroleum and Liquid
X = mole fraction of gas in equilibrium solution,
Petroleum Products by Hydrometer Method
L ,B = coefficients for mixture of gases,
m m
M = molecular weight of the gas, g/mol,
3. Terminology
M = molecular weight of the liquid, g/mol,
l
3.1 Definitions:
H = Henry’s law constant, MPa, and
3.1.1 Ostwald coeffıcient—the solubility of a gas expressed
C = molarity, kg mol/m .
as the volume of gas dissolved per volume of liquid when the
gas and liquid are in equilibrium at the specified partial
4. Summary of Test Method
pressure of gas and at the specified temperature.
4.1 Correlations have been established by the National
3.1.2 Bunsen coeffıcient—the solubility of a gas expressed
Aeronautics and Space Administration (formerly National
asthevolume,reducedto273K(32°F)and101.3kPa(1atm),
AdvisoryCommitteeonAeronautics)inNACATechnicalNote
dissolved by 1 volume of liquid at the specified temperature
3276 (1956) Their work was extended to include most of the
and 101.3 kPa.
data published since that time, and extrapolated by semi-
empirical methods into regions where no data are available.
1 4.2 Theonlydatarequiredarethedensityofliquidat288K
This test method is under the jurisdiction of ASTM Committee D02 on
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee (59°F) and the nature of the gas. These are used in the
D02.L0.07 on Engineering Sciences of High Performance Fluids and Solids.
equations, with the specific constant for the gas from Table 1,
Current edition approved May 1, 2007. Published June 2007. Originally
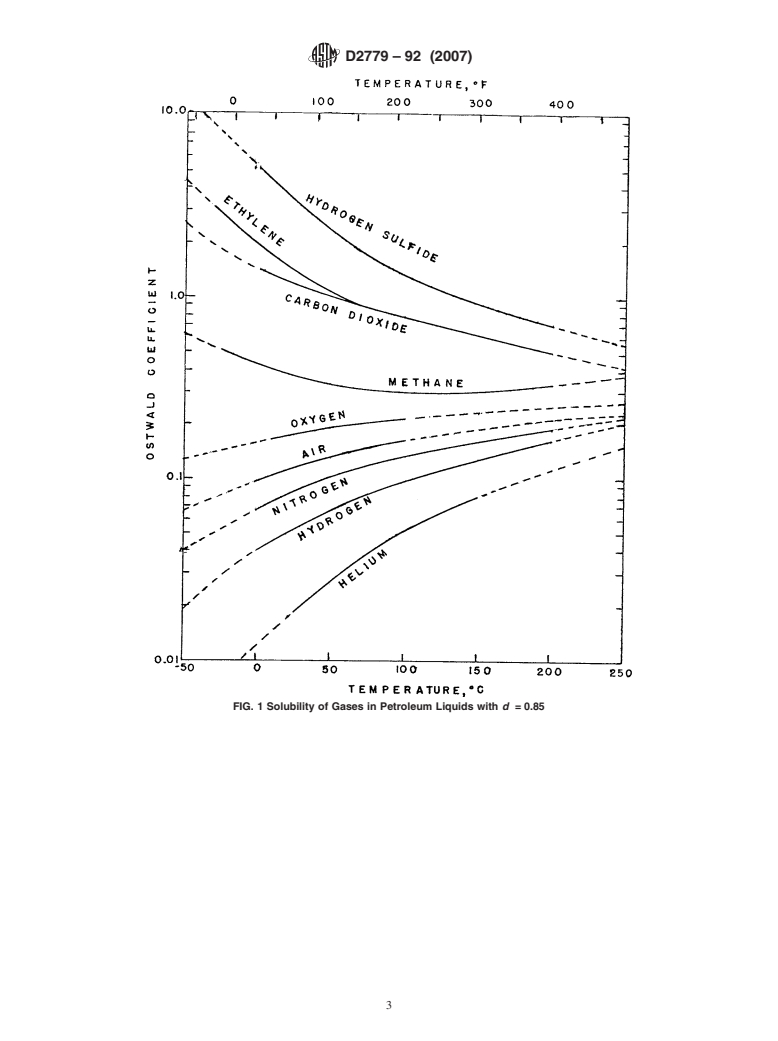
or with Fig. 1, to estimate the Ostwald coefficient.
approved in 1969. Last previous edition approved in 2002 as D2779–92 (2002).
DOI: 10.1520/D2779-92R07.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Available from National Aeronautics and Space Administration, Washington,
the ASTM website. DC.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2779–92 (2007)
TABLE 1 Ostwald Coefficients at 0°C for Petroleum Liquids with Itisalsothebestempiricalfitofthedata.Theuseofthisequationforvery
d =0.85
dense liquids is inadvisable, as the Ostwald coefficient becomes negative
A above d=0.980. The constant 7.70 is also predictable from molecular
Validated
Ostwald
theory, but the value used was determined empirically.
Gas Temperature
Coefficient, L
o
Range,° C
6.6 Calculate the Bunsen coefficient using the following
Helium 0.012 20–150
equation:
Neon 0.018 15–40
Hydrogen 0.040 0–200
B 52697 p– p L/T (3)
~ !
v
Nitrogen 0.069 0–200
Air 0.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.