ASTM D3612-02(2017)
(Test Method)Standard Test Method for Analysis of Gases Dissolved in Electrical Insulating Oil by Gas Chromatography
Standard Test Method for Analysis of Gases Dissolved in Electrical Insulating Oil by Gas Chromatography
SIGNIFICANCE AND USE
5.1 Oil and oil-immersed electrical insulation materials may decompose under the influence of thermal and electrical stresses, and in doing so, generate gaseous decomposition products of varying composition which dissolve in the oil. The nature and amount of the individual component gases that may be recovered and analyzed may be indicative of the type and degree of the abnormality responsible for the gas generation. The rate of gas generation and changes in concentration of specific gases over time are also used to evaluate the condition of the electric apparatus.
Note 1: Guidelines for the interpretation of gas-in-oil data are given in IEEE C57.104.
SCOPE
1.1 This test method covers three procedures for extraction and measurement of gases dissolved in electrical insulating oil having a viscosity of 20 cSt (100 SUS) or less at 40°C (104°F), and the identification and determination of the individual component gases extracted. Other methods have been used to perform this analysis.
1.2 The individual component gases that may be identified and determined include:
Hydrogen—H2
Oxygen—O2
Nitrogen—N2
Carbon monoxide—CO
Carbon dioxide—CO2
Methane—CH4
Ethane—C2H6
Ethylene—C2H4
Acetylene—C2H2
Propane—C3H8
Propylene—C3H6
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. For specific warning statements see 6.1.8, 30.2.2 and 30.3.1.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D3612 − 02 (Reapproved 2017)
Standard Test Method for
Analysis of Gases Dissolved in Electrical Insulating Oil by
Gas Chromatography
This standard is issued under the fixed designation D3612; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D2140Practice for Calculating Carbon-Type Composition
of Insulating Oils of Petroleum Origin
1.1 This test method covers three procedures for extraction
D2300Test Method for Gassing of Electrical Insulating
and measurement of gases dissolved in electrical insulating oil
Liquids Under Electrical Stress and Ionization (Modified
havingaviscosityof20cSt(100SUS)orlessat40°C(104°F),
Pirelli Method)
and the identification and determination of the individual
D2779Test Method for Estimation of Solubility of Gases in
component gases extracted. Other methods have been used to
Petroleum Liquids
perform this analysis.
D2780TestMethodforSolubilityofFixedGasesinLiquids
1.2 The individual component gases that may be identified 3
(Withdrawn 2010)
and determined include:
D3613Practice for Sampling Insulating Liquids for Gas
Hydrogen—H
2 AnalysisandDeterminationofWaterContent(Withdrawn
Oxygen—O 3
2007)
Nitrogen—N
D4051PracticeforPreparationofLow-PressureGasBlends
Carbon monoxide—CO
Carbon dioxide—CO
2 E260Practice for Packed Column Gas Chromatography
Methane—CH
2.2 IEEE Standard:
Ethane—C H
2 6
Ethylene—C H C57.104 GuidefortheInterpretationofGasesGeneratedin
2 4
Acetylene—C H
2 2
Oil-Immersed Transformers
Propane—C H
3 8
2.3 IEC Standard:
Propylene—C H
3 6
PublicationNo.567GuidefortheSamplingofGasesandof
1.3 This standard does not purport to address all of the
Oil from Oil-Filled Electrical Equipment and for the
safety concerns, if any, associated with its use. It is the
Analysis of Free and Dissolved Gases
responsibility of the user of this standard to establish appro-
priate safety, health, and environmental practices and deter-
3. Terminology
mine the applicability of regulatory limitations prior to use.
3.1 Definitions of Terms Specific to This Standard:
For specific warning statements see 6.1.8, 30.2.2 and 30.3.1.
3.1.1 gas content of oil by volume—in Method A, the total
1.4 This international standard was developed in accor-
volume of gases, corrected to 760 torr (101.325 kPa) and 0°C,
dance with internationally recognized principles on standard-
contained in a given volume of oil, expressed as a percentage.
ization established in the Decision on Principles for the
In Methods B and C, the sum of the individual gas concentra-
Development of International Standards, Guides and Recom-
tionscorrectedto760torr(101.325kPa)and0°C,expressedin
mendations issued by the World Trade Organization Technical
percent or parts per million.
Barriers to Trade (TBT) Committee.
3.1.2 headspace—a volume of gas phase in contact with a
2. Referenced Documents
volumeofoilinaclosedvessel.Thevesselisaheadspacevial
2.1 ASTM Standards: of 20-mL nominal capacity.
3.1.2.1 Discussion—Other vessel volumes may also be
used, but the analytical performance may be somewhat differ-
This test method is under the jurisdiction of ASTM Committee D27 on
ent than that specified in Method C.
Electrical Insulating Liquids and Gasesand is the direct responsibility of Subcom-
mittee D27.03 on Analytical Tests.
Current edition approved Nov. 15, 2017. Published December 2017. Originally
approved in 1977. Last previous edition approved in 2009 as D3612–02 (2009). The last approved version of this historical standard is referenced on
DOI: 10.1520/D3612-02R17. www.astm.org.
2 4
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Available from IEEE, 345 E. 47th St., New York, NY 10017.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Available from International Electrotechnical Commission (IEC), 3 rue de
Standards volume information, refer to the standard’s Document Summary page on Varembé, Case postale 131, CH-1211, Geneva 20, Switzerland, http://www.iec.ch.
the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3612 − 02 (2017)
3.1.3 parts per million (ppm) by volume of (specific gas) in 6. Apparatus
oil—thevolumeofthatgascorrectedto760torr(101.325kPa) 6
6.1 Apparatus of the type shown in Fig. 1 or Fig. 2 is
and 0°C, contained in 10 volume of oil.
suitable for use with up to 50-mL samples of oil and consists
3.1.4 sparging, v—agitatingtheliquidsampleusingagasto of the following components:
strip other gases free.
NOTE2—Thissamplesizehasbeenfoundtobesufficientformostoils.
However, oil that has had only limited exposure to air may contain much
3.1.5 volume concentration of (specific gas) in the gas
smalleramountsofnitrogenandoxygen.Fortheseoilsitmaybedesirable
sample—the volume of the specific gas contained in a given
to increase the size of the sample and the extraction apparatus.
volumeofthegassampleatthesametemperatureandpressure
NOTE 3—Alternative apparatus designs including the use of a Toepler
(as the measured total volume), expressed either as a percent-
pump have also been found successful.
age or in parts per million.
6.1.1 Polytetrafluoroethylene (PTFE) Tubing, narrow-bore,
terminatedwithaLuer-Lockfittedglasssyringe,andleadingto
4. Summary of Test Method
a solid plug, three-way, high-vacuum stopcock.
4.1 MethodA—Dissolvedgasesareextractedfromasample 6.1.2 Degassing Flask, with a glass inlet tube, of sufficient
of oil by introduction of the oil sample into a pre-evacuated volume to contain up to 50 mL of oil below the inlet tube,
capable of being evacuated through a vacuum pump, contain-
known volume. The evolved gases are compressed to atmo-
spheric pressure and the total volume measured. ing a PTFE-coated magnetic spin bar, and mounted on a
magnetic stirrer.
4.2 Method B—Dissolvedgasesareextractedfromasample
6.1.3 Means of Measuring Absolute Pressure within the
of oil by sparging the oil with the carrier gas on a stripper
apparatus.
column containing a high surface area bead.
6.1.4 Vacuum Pumping System, capable of evacuating the
−3
4.3 Method C—MethodCconsistsofbringinganoilsample
glasswaretoanabsolutepressureof1×10 torr(130mPa)or
in contact with a gas phase (headspace) in a closed vessel
lower.
purgedwithargon.Thedissolvedgasescontainedintheoilare
6.1.5 Vacuum Glassware, sufficiently large compared to the
then equilibrated in the two phases in contact under controlled
volume of the oil sample, so that virtually complete degassing
conditions (in accordance with Henry’s law). At equilibrium,
isobtainedandthatthevolumetriccollectionratioisaslargeas
the headspace is overpressurized with argon and then the
possible. A 500-mL gas collecting flask has been found
content of a loop is filled by the depressurization of the
suitable.
headspaceagainsttheambientatmosphericpressure.Thegases
6.1.6 High-Vacuum Valves or Stopcocks, employing the
contained in the loop are then introduced into a gas chromato-
minimum necessary amounts of high-vacuum stopcock grease
graph.
are used throughout the apparatus.
6.1.7 Gas Collection Tube, calibrated in 0.01-mLdivisions,
4.4 Theremaybesomedifferencesinthelimitsofdetection
capable of containing up to 5 mL of gas, terminated with a
and precision and bias between Methods A, B, and C for
silicone rubber retaining septum. A suitable arrangement is
various gases.
shown in Fig. 3.
4.5 Aportionoftheextractedgases(MethodA)orallofthe
6.1.8 Reservoir of Mercury, sufficient to fill the collection
extractedgases(MethodB)oraportionoftheheadspacegases
flask and collection tube. (Warning—Mercury vapor is ex-
(Method C) is introduced into a gas chromatograph. Calibra-
tremely toxic. Appropriate precautions should be taken.)
tioncurvesareusedinMethodCtoestablishtheconcentration
of each species. The composition of the sample is calculated 7. Sampling
from its chromatogram by comparing the area of the peak of
7.1 Obtain samples in accordance with the procedure de-
each component with the area of the peak of the same
scribed in Test Methods D3613 for sampling with syringetype
component on a reference chromatogram made on a standard
devices or rigid metal cylinders. The use of rigid metal
mixture of known composition.
cylinders is not recommended for use with Method B.
7.2 The procurement of representative samples without loss
5. Significance and Use
ofdissolvedgasesorexposuretoairisveryimportant.Itisalso
5.1 Oilandoil-immersedelectricalinsulationmaterialsmay
important that the quantity and composition of dissolved gases
decompose under the influence of thermal and electrical
remain unchanged during transport to the laboratory. Avoid
stresses, and in doing so, generate gaseous decomposition
prolonged exposure to light by immediately placing drawn
products of varying composition which dissolve in the oil.The
samples into light-proof containers and retaining them there
natureandamountoftheindividualcomponentgasesthatmay
until the start of testing.
be recovered and analyzed may be indicative of the type and
7.2.1 To maintain the integrity of the sample, keep the time
degree of the abnormality responsible for the gas generation.
between sampling and testing as short as possible. Evaluate
The rate of gas generation and changes in concentration of
specific gases over time are also used to evaluate the condition
of the electric apparatus. Ace Glass and Lurex Glass manufacture glass extractors. For Ace Glass, the
glass apparatus conforming to Fig. 1 is Part E-13099-99-99 and Fig. 2 is Part
NOTE1—Guidelinesfortheinterpretationofgas-in-oildataaregivenin E-1400-99. Available from P.O. Box 688, 1430 Northwest Blvd., Vineland, NJ
IEEE C57.104. 08360 or Lurex Glass, 1298 Northwest Blvd., Vineland, NJ 08360.
D3612 − 02 (2017)
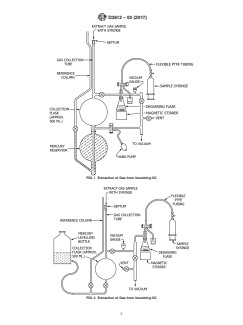
FIG. 1 Extraction of Gas from Insulating Oil
FIG. 2 Extraction of Gas from Insulating Oil
D3612 − 02 (2017)
9.5 Ostwald solubility coefficients that have been deter-
mined for a number of gases in one specific electrical insulat-
ing oil at 25°C are shown as follows.Values for gases in other
oils may be estimated by reference to Test Method D2779.
Ostwald Solubility (Note 5)
Component Gas
Coefficient, K , 25°C, 760 mm Hg
i
Hydrogen 0.0558
Nitrogen 0.0968
Carbon monoxide 0.133
Oxygen 0.179
Methane 0.438
Carbon dioxide 1.17
Acetylene 1.22
Ethylene 1.76
Ethane 2.59
Propane 11.0
NOTE 5—The Ostwald coefficient values shown in this table are correct
onlyforthespecificmineraloilhavingadensityat15.5°Cof0.855g/cm
usedintheoriginaldetermination.Ostwaldcoefficientsformineraloilsof
different density may be calculated as follows:
FIG. 3 Retaining Rubber Septum for Gas Collection Tube
0.980 2density
K ~corrected! 5 K (3)
i i
0.130
where, density =density of the oil of interest, g/cm at 15.5°C (60°F).
containers for maximum storage time. Samples have been
This equation is derived from the equation in Test Method D2779. Note
stored in syringes and metal cylinders for four weeks with no
especially that all of the Ostwald coefficients are changed by the same
factor, meaning that though the absolute solubilities of each of the gases
appreciable change in gas content.
will change if a different oil is used, the ratio of the solubility of one gas
NOTE 4—Additional sampling procedures using flexible metal cans are
to another gas will remain constant.
currently being studied for use with Method A.
9.6 A procedure to check the extraction efficiency requires
the use of prepared gas-in-oil standards of known concentra-
METHOD A—VACUUM EXTRACTION
tion.ThemethodsofpreparationareoutlinedinAnnexA1and
8. Method A—Vacuum Extraction
Annex A2.
8.1 Method A employs vacuum extraction to separate the
10. Procedure
gases from the oil. The evolved gases are compressed to
atmospheric pressure and the total volume measured. The
10.1 Lower the mercury level from the collection flask.
gases are then analyzed by gas chromatography.
10.2 Evacuate the system of collection flask and degassing
−3
flasktoanabsolutepressureof1×10 torr(130mPa)orless.
9. Preparation of Apparatus
(In Fig. 1, the space above the mercury in the reservoir must
9.1 Check the apparatus carefully for vacuum tightness of
also be evacuated.)
all joints and stopcocks.
10.3 Connect the oil sample syringe by the PTFE tubing to
9.2 Measure the total volume of the extraction apparatus,
the three-way stopcock leading to the degassing flask.
V , and the volume of the collection space, V , and calculate
T c
10.4 Flush a small quantity of oil from the syringe through
the ratio as the volumetric collection ratio:
thetubingandstopcocktowaste,makingsurethatalltheairin
V
c
the connecting tubing is displaced by oil.
(1)
V 2 V
T o
10.4.1 Any gas bubbles present in the syringe should be
retained during this flushing operation. This may be accom-
where V =the volume of oil to be added.
o
plished by inverting the syringe so that the bubble remains at
9.3 Calculate the degassing efficiencies for each individual
the plunger end of the syringe during the flushing operation.
component gas as follows:
10.5 Close the stopcocks to the vacuum pumps and then
slowly open the three-way stopcock to allow oil and any gas
E 5 (2)
i
K V
i o
bubbles that may be present from the sample syringe to enter
V 2 V
T o
the degassing flask.
where:
10.6 Allow the desired amount of oil to enter the degassing
E = degassing efficiency of component i,
flask and operate the magnetic stirrer vigorously for approxi-
i
V = volume of oil sample,
o mately 10 min. This is the volume, V used in the calculation
o
V = totalinternalvolumeofextractionapparatusbeforeoil
T in 15.4.
sample is introduced, and
K = Ostwald solubility coefficient of component i.
i
9.4 Determine the Ostwald solubility coefficients of fixed
Daoust, R., Dind, J. E., Morgan, J., and Regis, J, “Analysis of Gas Dissolved
gases in accordance with Test Method D2780. in Transformer Oils,” Doble Conference, 1971, Sections 6–110.
D3612 − 02 (2017)
10.6.1 If a gas bubble is present in the syringe, either 11.5 Awiderangeofchromatographicconditionshavebeen
analyze the total content of the syringe including the bubble; successfullyemployed.Bothargonandheliumhavebeenused
or, if the gas bubble is large, and it is suspected that the as carrier gases (see Note 7). In some cases, a separate GC or
concentration of dissolved gases is high, measure and analyze other device is used for the detection and quantification of
the gas bubble separately, extract an aliquot of the oil sample, hydrogen when helium is used as a carrier gas.
and correct as applicable.
NOTE 7—If helium is used as a carrier gas with a thermal conductivity
detector,mediumtohighconcentrationsofhydrogenmaygiveanonlinear
10.7 Close the stopcock isolating the collection flask, and
response, due to the closed heat capacity values of helium and hydrogen.
allow mercury to flow into the collection flask.
The limit of detection will be higher than with an argon carrier gas under
similar conditions. If nitrogen is used as a carrier gas, nitrogen cannot be
10.8 Open the stopcock to the reference column and by
detected in the sample.
means of the hand pump (Fig. 1) or leveling bottle (Fig. 2)
11.5.1 With the use of an argon carrier gas, a catalytic
bring the level of the mercury in the reference column even
converter containing powdered nickel located after the chro-
with the level in the collection tube.
matographic columns is used to convert carbon monoxide and
10.9 Measure the volume of extracted gas in the collection
carbondioxidetomethanefordetectionwithaflameionization
tube, and correct for collection efficiency by dividing it by the
detector for acceptable sensitivity. (The condition of the nickel
volumetriccollectionratiocalculatedin9.2.Correctto760torr
catalyst can be evaluated by checking the linearity of the
(101.325 kPa) and 0°C. Determine the volume of oil degassed
response to carbon dioxide.) With helium as a carrier gas, a
in the degassing flask. Record the gas content as a percentage
catalyticconverterisnotnecessarybutmaybeusedtoenhance
of the oil by volume.
sensitivity.
11.5.2 A flame ionization detector, instead of a thermal
10.10 Because the total concentration of gas is not extract-
conductivitydetector,isoftenusedtodetecthydrocarbongases
able from the oil, a rinse step may be required when high
due to its greater sensitivity for these components. A wide
quantities are present. The extractor can be rinsed with oil
range of injector, column, and detector temperatures can be
containing nondetectable quantities of gases, except for those
used.Bothisothermalandtemperatureprogramscanbeusedto
present in air.The amount of rinsing needed will be dependent
provide adequate separation and sensitivity. A typical chro-
upon the gas concentration, type (solubility in oil), and
matogram is shown in Fig. 4.
efficiencyoftheextractor.Toensurethatthecombustiblegases
havebeensufficientlyremovedfromtheextractor,therinseoil
11.6 Fixed Needle Gas-Tight Syringes ,ofsuitablesizesare
may be treated as a sample. General rinse procedures may be
needed for transfer of the gases.
established. However, for samples with very high concentra-
tions of gases, verify effectiveness of the rinse procedure.
12. Reagent and Materials
12.1 Purity of Reagents—Reagent grade chemicals shall be
GAS ANALYSIS
used in all tests. Unless otherwise indicated, it is intended that
all reagents shall conform to the specifications of the Commit-
11. Apparatus
tee onAnalytical Reagents of theAmerican Chemical Society,
11.1 Gas Chromatograph, consisting essentially of a carrier
where such specifications are available. Other grades may be
gas source, a pressure regulator, a sample injection port and
used, provided it is first ascertained that the reagent is of
chromatography column(s), flow meter(s), detector(s), and
sufficiently high purity to permit its use without lessening the
recorder(s) or recording integrator(s).
accuracy of the determination.
11.2 Provide means for measuring and controlling tempera-
12.2 Suitable Chromatography Columns—Several combi-
tures of the adsorption column, the inlet port, and the detector
nations have been found to be suitable, including molecular
to within 60.5°C.
sieve,PorapakQ,PorapakS,diisodecylphthalateA,SilicaGel
J, Chromosorb 102, and Carbosieve B.
NOTE 6—Use Practice E260 as a reference for good chromatographic
techniques.
12.3 Helium, Argon, or Nitrogen Carrier Gas, having a
minimum purity of 99.95 mol % (see Note 7).
11.3 The apparatus shall be capable of sufficiently separat-
ing the component gases, at the sensitivity levels shown as 12.4 Reference Standard Gas Mixture, containing known
follows, to ensure quantitative measurement of the respective percentages of the gases shown in 11.3.
peak areas:
Minimum Detection Limits for Gases
Component Gas
Dissolved in Oil, ppm
Syringes that have been found suitable include those from the Hamilton Co.,
Hydrogen 5
P.O. Box 307, Whittier, CA 90608; Pressure-Lok Syringes made by Precision
Hydrocarbons 1
Sampling Corp., P.O. Box 15119, Baton Rouge, LA 70815; and Popper and Sons,
Carbon oxides 25
Inc., 300 Denton Ave., New Hyde Park, NY 11040.
Atmospheric gases 50
Reagent Chemicals, American Chemical Society Specifications , American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
11.4 The apparatus shall provide sufficient repeatability so
listed by the American Chemical Society, see Analar Standards for Laboratory
thatsuccessiverunsofareferencestandardagreewithin 61%
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
with respect to area under the peaks for hydrocarbon and
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
carbon oxide components. MD.
D3612 − 02 (2017)
Gas Chromatograph Conditions:
Argon carrier gas, flow rate 30 mL/min
Columns: Porapak N, 80–100 mesh, 13 ft × ⁄8 in.
Molecular sieve, 13×, 40–60 mesh, 3 ft × ⁄8 in.
Catalytic converter for detection of CO and CO
Detectors: Thermal conductivity: H ,O ,N
2 2 2
Flame ionization: CH , CO, CO ,C H ,C H ,C H ,C H ,C H ,C H
4 2 2 6 2 4 2 2 3 8 3 6 4 10
Temperatures: Injection 200°C
TCD 150°C
FID 300°C
Column: Isothermal 35°C for 8 min
35–132°C ramp at 20°C/min, hold until 15.5 min
132–150°C ramp at 25°C/min, hold
NOTE 1—Propane and propylene are not separated under these conditions.
FIG. 4 Sample Chromatogram
12.4.1 Aroundrobinperformedforthistestmethodshowed million. Normally, the gas standard is prepared at concentra-
considerable variation in gas standards when compared to a tions of 5 to 10 times that seen in the oil due to the
supplied primary standard. It is strongly recommended that concentration effect of extracting the gas from the oil and
only primary standards (each component prepared gravimetri- because higher concentrations can be prepared with greater
cally) be used. Refer to Practice D4051 for procedures used to accuracy. Some laboratories use more than one concentration
prepare a blend of standard gases. The National Institute of of standards.Acetylene is of greater concern at lower concen-
Standards and Technology (NIST) has some gas standards tration levels than the other hydrocarbon gases.
available which can be used to calibrate working standards.
13. Calibration
12.4.2 Individual gases can range from detectable levels to
13.1 Prepare the gas chromatograph for use as directed by
thousands of parts per million in actual samples. However, in
the manufacturer, and establish a set of operating conditions
most samples the concentration of gases (except oxygen,
capable of separation of the indicated component gases.
nitrogen, and carbon dioxide) is tens to hundreds of parts per
13.2 Inject a pre-established volume of the reference stan-
dardgasmixtureintothechromatographandestablishapattern
AvailablefromU.S.DepartmentofCommerce,NationalInstituteofStandards
of elution times for the gas components known to be in the
and Technology, Standard Reference Materials Program, Bldg. 202, Room 204,
Gaithersburg, MD 20899. mixture, at an established set of operating conditions and
D3612 − 02 (2017)
sample size. Repeat the analysis until consistent operating
T = ambient temperature, in Kelvin.
a
conditions provide consistent chromatograms as specified in
15.5 Correct each experimental value obtained in 15.4 for
11.4. Repeat calibration daily when analyses are being con-
incomplete degassing by dividing each value by its respective
ducted.
degassing efficiency derived from 9.3.
C
14. Procedure i
(5)
E
i
14.1 Increase the pressure on the extracted gas contained in
the collection tube, described in 6.1.7 to slightly above
16. Report
atmospheric pressure by raising the level of mercury in the
16.1 Report the following information:
referencecolumnslightlyabovethelevelofmercuryinthegas
16.1.1 Identification of oil sample,
collection tube.
16.1.2 Temperature of oil at time of sampling,
14.2 Insert the needle of the gas-tight injection syringe
16.1.3 Gas content of oil by volume, expressed as a
through the septum of the collection tube, and withdraw a
percentage,
suitablevolumeofgasintothesyringe.Adjustthegaspressure,
16.1.4 Volume concentration in the oil, for each component
as indicated by the reference column, precisely to atmospheric
gas, expressed in parts per million, and
pressure before closing the syringe or withdrawing the needle
16.1.5 Test method used (for example, D3612, Part A).
from the septum.
14.3 When the apparatus conditions are equal to those
17. Precision and Bias
established during the calibration procedure, quickly inject the
17.1 The precision, bias and lower limit of detection of
known volume of gas into the chromatograph through the
Method A have been evaluated by a statistical examination of
injection port.
the results of an inter-laboratory test of mineral oil test
14.4 Periodically, chromatography columns require baking 11
specimens. A lower limit of repetition is defined here as an
out at elevated temperatures. The frequency and duration will
aid in the testing of transformers in factories.
depend upon such factors as type of column, amount of use,
17.2 Precision – Repeatability—The expected difference
and concentration of materials tested. Peaks which are not as
betweensuccessiveresultsobtainedonidenticaltestspecimens
sharpasusualmaybefromcompoundsretainedonthecolumn
bythesameoperatorusingthesameapparatusandnormaland
fromapreviousrun,andmayindicateaneedforbakingoutthe
correct operation of the test method.
columns. Another indicator that the molecular sieve column
17.2.1 Combustible Gases and Carbon Dioxide—
needs conditioning is that the methane and carbon monoxide
Repeatability of the determination of each individual combus-
peaks begin to lose baseline separation.
tiblegasandofcarbondioxidewasfoundtovarylinearlywith
individual gas concentration level.The repeatability interval at
15. Calculation
the 95% confidence level for the determination of a combus-
15.1 Determine the integrated area of each peak of the
tible gas n or of CO,l (r) can be represented by:
2 n 95%
chromatogram.
l ~r! 5 k ~r! 3C (6)
n n n
95% 95%
15.2 Identify the gases represented by each peak by com-
parison of elution times with those obtained for the reference where l (r) isthevalueoftherepeatabilitycoefficientfor
n 95%
thedeterminationofthatcombustiblegasorofcarbondioxide.
standard gas mixture in the calibration procedure.
C is the concentration level of the gas of interest (ppm). The
n
15.3 Determine the amount of each identified gas compo-
repeatability coefficients at the 95% level for each of the
nent by comparing respective peak areas with those obtained
combustible gases and for CO and the concentration ranges
for the reference standard gas mixture in the calibration
tested are given in Table 1.
procedure.
17.2.2 Oxygen and Nitrogen—The ranges of concentrations
15.4 Calculate the volume concentration of each specific
of oxygen and nitrogen in the test specimens analyzed in the
gaswithrespecttothevolumeofoildegassedinthedegassing
inter-laboratory test were relatively narrow. Therefore the
flask. Correct to 760 torr (101.325 kPa) and 0°C, and express
relationships between repeatability intervals and concentra-
as parts per million of (specific gas) in oil, by volume.
tions of dissolved O or of N are not well defined. The
2 2
coefficients of variation, S(r), at the 50% confidence level for
V A C P 273 310
g i si a
C 5 (4)
i
A V 760 T the repeatability of the determination of O and of N and the
si o a 2 2
concentration ranges tested are given in Table 1.
where:
17.3 Precision – Reproducibility—The expected difference
V = volume of gas extracted,
g
between two results obtained on identical test specimens by
C = concentration of gas in ppm, vol/vol,
i
different operators working in different laboratories under
A = area count or peak height for gas i in sample,
i
normal and correct operation of the test method.
A = area count or peak height for gas i in standard,
si
C = concentration of gas i in standard in percent vol/vol,
si
V = volume of oil,
o
P = atmospheric pressure, in torr, and
a 11
Available from ASTM Headquarters. Request RR:D27-1016.
D3612 − 02 (2017)
TABLE 1 Summary of Precision and Bias for Method A
shown graphically in Fig. 5. It is possible that the positive bias
Gas C° - Range Repeatability Reproducibility Bias at lower concentrations results, in part, from contamination by
air.
Combustible Gases and Carbon Dioxide
17.4.3 Oxygen and Nitrogen—The bias for determinations
n ppm k (r) k (R) B
n 95 % n 95 % n
H 90 – 710 0.31 0.38 −0.13 of O and of N are positive and variable. It is possible that
2 2
CO 110 – 930 0.28 0.79 −0.14
positivebiasis,inpart,theresultofcontaminationbyair.Also,
CH 35 – 620 0.25 0.72 −0.21
the ranges of concentrations of oxygen and of nitrogen in the
C H 40 – 400 0.37 0.75 −0.29
2 6
C H 30 – 800 0.28 0.82 −0.27
test specimens analyzed in the interlaboratory test were rela-
2 4
C H 25 – 335 0.29 0.64 −0.30
2 2
tively narrow. The relationships between bias and dissolved
A
CO 45 – 9300 0.48 0.76
concentration of O or of N then are not well defined. The
Oxygen and Nitrogen 2 2
n ppm S (r) S (R) B
coefficients of variation S(R) at the 50% confidence level for
n 50 % n 50 % n
O 4630 – 4670 0.25 0.35 0.07 – 0.53
thereproducibilityofthedeterminationofO andofN andthe
2 2
N 27000 – 61000 0.14 0.27 0.47 – (−) 0.05
concentration ranges tested are given in Table 1.
A
See text.
METHOD B—STRIPPER COLUMN EXTRACTION
18. Method B—Stripper Column Extraction
17.3.1 Combustible Gases and Carbon Dioxide—
18.1 Dissolved gases are extracted from a sample of oil by
Reproducibility of the determination of each individual com-
sparging the oil with the carrier gas on a stripper column
bustible gas and of carbon dioxide was found to vary linearly
containingahighsurfaceareabead.Thegasesarethenflushed
with individual gas concentration level. The reproducibility
from the stripper column into a gas chromatograph for analy-
interval at the 95% confidence level for the determination of a
sis. Testing of silicone liquids by this test method is not
combustible gas n or of CO,l (R) can be represented by: recommended for systems which are also used to test mineral
2 n 95%
oil, as excessive foaming should cause contamination of
l ~R! 5 K ~R! 3C (7)
n n n
95% 95%
columns after the stripper.
where l (R) is the value of the reproducibility coefficient
n 95%
19. Apparatus
for the determination of that combustible gas or of carbon
dioxide. C is the concentration level of the gas of interest
n 19.1 Gas Chromatograph , capable of separating and de-
(ppm). The reproducibility coefficients at the 95% level for
tectingthegasesofinterestusingadirectinjectionofaportion
each of the combustible gases and CO and the concentration
2 oftheliquidsamples.AlternativegasstrippersaregiveninIEC
ranges tested are given in Table 1.
Guide567.
17.3.2 Oxygen and Nitrogen—The ranges of concentrations
19.2 The apparatus must be capable of sufficiently separat-
of oxygen and of nitrogen contained were relatively narrow in
ing the component gases, at the sensitivity levels shown as
the specimens analyzed in the interlaboratory test. Therefore
follows, to ensure quantitative measurement of the respective
therelationshipsbetweenreproducibilityintervalsandconcen-
peak areas:
tration of dissolved O or N are not well defined. The
2 2
Minimum Detection Limits for Gases Dis
coefficients of variation, S(R), at the 50% confidence level for
Component Gas
solved in Oil, ppm
thereproducibilityofthedeterminationofO andofN andthe
2 2 Hydrogen 20
concentration ranges tested are given in Table 1. Hydrocarbons 1
Carbon oxides 2
17.4 Bias—The difference between the mean of results
Atmospheric gases 500
obtained for a gas in a test specimen and the “true” (that is,
ThelimitofdetectionforhydrogenspecifiedinMethodBis
spiked) value of the concentration of that gas in the tested
higher than that specified for Method A. This could affect the
material.
interpretation of results when low levels of gases are present.
17.4.1 Combustible Gases—Bias of the determination of
19.3 The apparatus shall be capable of providing data for
each individual combustible gas was found to vary linearly
successive runs of a reference standard that are repeatable
with individual gas concentration level. The relative bias, B ,
n
within 1%, with respect to area under the peaks, for hydrogen
for the determination of a combustible gas, n, can be repre-
and carbon oxide components.
sented by:
o o
B 5 C 2 C /C (8) 20. Reagent and Materials
~ !
n n n n
20.1 Suitable Chromatography Columns— Several combi-
where C is the concentration level of the gas of interest
n
o
nations have been found to be suitable including molecular
(ppm) and C is the “true” (spiked) value of the concentration
n
sieve,PorapakQ,PorapakN,diisodecylphthalateA,SilicaGel
of that gas in that test material. The bias and the concentration
J, Chromosorb 102, Carbosieve B, and Sperocarb. Molecular
ranges tested are given in Table 1 for each of the combustible
sieve is used to separate H,O,N,CH , and CO. Porapak N,
gases.ThebiasesinresultsfromMethodAforthecombustible
2 2 2 4
gases are uniformly negative.
17.4.2 Carbon Dioxide—Bias for the determination of car-
Suitable equipment includes that from Shimadzu Scientific Instruments, Inc.,
bon dioxide decrease with increasing CO . No analytical
2 7102 Riverwood Road, Columbia, MD.This equipment uses a patented process for
transformation adequately fits the results; these results are the sparger.
D3612 − 02 (2017)
NOTE 1—Co = Calculated CO
C = Average of CO Method A
A 2
C = Average of CO Method B
FIG. 5 CO in Oil — D3612 A&B Interlaboratory Test - Average Result versus Nominal Concentration
Q, or combinations of both are used to separate CO,C H , 20.2 Argon, or Nitrogen Carrier Gas, having a minimum
2 2 4
C H,C H,C H,C H , and C H . Sperocarb is used to purity of 99.95 mol % with total hydrocarbons of less than 0.5
2 6 2 2 3 6 3 6 4 10
separate the carbon oxide and hydrocarbon gases. ppm and CO of less than 1 ppm. (See Note 7.)
D3612 − 02 (2017)
20.2.1 With the use of an argon carrier gas, a catalytic 22.3 Determine the linearity of the detector response
converter containing powdered nickel, located after the sepa- monthlybytestingarangeofgasconcentrationsexpectedtobe
ratingcolumns,isusedtoconvertcarbonmonoxideandcarbon encountered in actual samples. Extraction efficiencies should
dioxide to methane for detection with a flame ionization also be determined over a corresponding range to ensure they
detector for acceptable sensitivity. (The condition of the nickel are linear and constant over time. Samples can be prepared by
catalyst can be evaluated by checking the linearity of the simpledilutionofpuregaseswitheithernitrogenorcarriergas
response to carbon dioxide.) (for gas standards) or degassed oil (for gas-in-oil standards). If
commercially supplied standard mixtures are used, they may
20.3 Flame Ionization Detector Gases—Hydrogen having a
be checked using this method. Check efficiencies and linearity
purity of 99.99 mol % with total hydrocarbons of less than 0.5
whenever chromatographic conditions are changed.
ppm and air having a purity of less than 1 ppm total
hydrocarbons.
23. Procedure for Direct Injection
20.4 Reference Standard Gas Mixtures—Low-concentration
23.1 Prepare the gas chromatograph as outlined by the
standard containing known percentages of the gases in 1.2 at
manufacturer.
concentrations approximately the magnitude of the values
normally encountered. The high-concentration gas standard 23.2 Prepare the sample for injection by first dissolving any
gas bubble present into the volume of oil by compressing the
should contain levels approximately one order of magnitude
higher than contained in the low-concentration gas standard. plunger into the barrel of the syringe and agitating the gas by
tipping the syringe up and down. Any bubble present in the
The gas standards should be a primary grade (each component
added gravimetrically). The high gas standard is used for syringe must be dissolved to obtain a representative aliquot of
the sample for injection. Small volumes of oil are needed for
preparing gas in oil standards as outlined in Annex A1.
flushing and sample, typically a total of several millitres.
21. Calibration (Gases)
Flushing is required to displace the previous sample from the
21.1 Prepare the gas chromatograph for use as directed by
column.
the manufacturer, and establish a set of operating conditions
23.3 Once the sample is connected to the gas
capable of separating the indicated component gases.
chromatograph, flush enough oil through the injection system
21.2 Inject a preestablished volume of the reference stan-
to ensure that no gas bubbles remain in the line.
dard (low concentration) gas mixture into the chromatograph
23.4 If high concentrations of the more soluble gases are
and establish a pattern of elution times for the gas components
found, in particular C H , the injection column can be back
2 2
known to be in the mixture, at an established set of operating
flushed.Useablankrunofdegassedinsulatingoiltocheckthat
conditions and sample sizes. Repeat the analysis until consis-
no residual gases remain.
tent operating conditions provide consistent chromatograms.
Repeat calibration daily when analyses are being conducted.
24. Calculation
22. Efficiency Determination
24.1 Determine the integrated area of each peak of the
chromatogram.
22.1 Inject the oil standard prepared from one of the
procedures in the Annexes into the system. Determine the
24.2 Identify the gases represented by each peak by com-
dissolved gas content of this oil chromatographically based
parison of elution times with those obtained for the reference
upon the low-concentration gas standard. The difference be-
standard gas mixture in the calibration procedure.
tween the calculated concentration and the observed concen-
24.3 Determine the amount of each identified gas compo-
tration is the degassing efficiency of a given component and
nent by comparing respective peak areas with those obtained
may be calculated as follows:
for the reference standard gas mixture in the calibration
D C 2 C /C (9)
~ !
i aoi boi oi
procedure.
where: 24.4 Correct the values obtained based on the efficiency
values obtained in the efficiency determination procedure, and
D = degassing efficiency of component i,
i
express as parts per million of (specific gas) in oil, by volume
C = observed concentration of component i in the oil
aoi
as shown in the following calculation:
standard,
C = observed concentration of component i in the blank
boi
C 5 C /D (10)
ci aoi i
oil, and
where:
C = calculated concentration of component i in the oil
oi
standard.
C = observed concentration of component i in the oil
aoi
sample, and
22.2 The degassing efficiency factor is used to correct the
C = corrected concentration of component i in the oil
ci
determined concentration values for incomplete extraction.
sample.
Repeat the procedure until consistent results are obtained.
Conduct this efficiency determination weekly for at least one
25. Report
concentration of standard gas. Whenever there are changes in
the chromatographic system, redetermine the extraction effi- 25.1 Report the following information:
ciency. 25.1.1 Identification of oil sample,
D3612 − 02 (2017)
25.1.2 Temperature of oil at time of sampling, 26.3.1 Combustible Gases and Carbon Dioxide—
25.1.3 Volume concentration in the oil, for each component Reproducibility of the determination of each individual com-
gas, expressed in parts per million, and bustible gas and of carbon dioxide was found to vary linearly
25.1.4 The test method used (for example, D3612, Part B). with individual gas concentration level. The reproducibility
interval at the 95% confidence level for the determination of a
26. Precision and Bias
combustible gas n or of CO , l ( R) can be represented by:
2 n 95%
26.1 The precision, bias and lower limit of detection of
l ~R! 5 k ~R! 3C (12)
n n n
95% 95%
Method B have been evaluated by a statistical examination of
where l (R) is the value of the reproducibility coefficient
the results of an inter-laboratory test of mineral oil test n 95%
for the determination of that combustible gases or of carbon
specimens. A lower limit of repetition is defined here as an
dioxide. C is the concentration level of the gas of interest
aid in the testing of transformers in factories. n
(ppm). The reproducibility coefficients at the 95% level for
26.2 Precision – Repeatability—The expected difference
each of the combustible gases and CO and the concentration
betweensuccessiveresultsobtainedonidenticaltestspecimens
ranges tested are given in Table 2.
bythesameoperatorusingthesameapparatusandnormaland
26.3.2 Oxygen and Nitrogen—The ranges of concentrations
correct operation of the test method.
of oxygen and of nitrogen contained were relatively narrow in
26.2.1 Combustible Gases and Carbon Dioxide—
the specimens analyzed in the inter-laboratory test. Therefore
Repeatability of the determination of each individual combus-
therelationshipsbetweenreproducibilityintervalsandconcen-
tiblegasandofcarbondioxidewasfoundtovarylinearlywith
tration of dissolved O or N are not well defined. The
2 2
individual gas concentration level.The repeatability interval at
coefficients of variation, S(R), at the 50% confidence level for
the 95% confidence level for the determination of a combus-
thereproducibilityofthedeterminationofO andofN andthe
2 2
tible gas n or of CO,l (r) can be represented by:
2 n 95%
concentration ranges tested are given in Table 2.
l r 5 k r 3C (11)
~ ! ~ !
n n n
95% 95%
26.4 Bias—The difference between the mean of results
obtained for a gas in a test specimen and the “true” (that is,
where l (r) isthevalueoftherepeatabilitycoefficientfor
n 95%
spiked) value of the concentration of that gas in the tested
the determination of that combustible gases or of carbon
material.
dioxide. C is the concentration level of the gas of interest
n
26.4.1 Combustible Gases—Bias of the determination of
(ppm).The repeatability coefficients at the 95% level for each
each individual combustible gas was found to vary linearly
of the combustible gases and for CO and the concentration
with individual gas concentration level. The relative bias, B ,
ranges tested are given in Table 2. n
for the determination of a combustible gas, n, can be repre-
26.2.2 Oxygen and Nitrogen—The ranges of concentrations
sented by:
ofoxygenandofnitrogeninthetestspecimensanalyzedinthe
o o
interlaboratory test were relatively narrow. Therefore the
B 5 C 2 C /C (13)
~ !
n n n n
relationships between repeatability intervals and concentra-
where C is the concentration level of the gas of interest
n
tions of dissolved O or of N are not well defined. The
2 2 o
(ppm) and C is the “true” (spiked) value of the concentration
n
coefficients of variation, S(r), at the 50% confidence level for
of that gas in that test material. The bias and the concentration
the repeatability of the determination of O and of N and the
2 2
ranges tested are given in Table 3 for each of the combustible
concentration ranges tested are given in Table 2.
gases.
26.3 Precision – Reproducibility—The expected difference
NOTE 8—The distributions of results for the determination of hydrogen
between two results obtained on identical test specimens by
byMethodBarebipolar(seeFig.6).Theresultsfromtwelvelaboratories
differentoperatorsworkingindifferentlaboratoriesandnormal
form primary nodes centered about the “true” concentrations of test
and correct operation of the test method.
specimens. The biases reported in Table 2 are based on these primary
nodes. The results from five labs form secondary nodes at or near
concentrations of zero. This emphasizes the need for a routine QA
protocol for the determination of H2 by Method B.
TABLE 2 Summary of Precision and Bias for Method B
Gas C° - Range Repeatability Reproducibility Bias
Combustible Gases and
Carbon Dioxide
n ppm k (r) k (R) B TABLE 3 Lower Limits —Detection and Repetition Method B
n 95 % n 95 % n
H 90 – 710 0.17 0.61 −0.07
NOTE 1—Better MRLs may be achieved by individual labs that can
CO 110 – 930 0.17 0.51 0.02
demonstrate better repeatability than the interlaboratory test study sug-
CH 35 – 620 0.08 0.61 −0.03
gests.
C H 40 – 400 0.08 0.86 0.00
2 6
C H 30 – 800 0.09 0.76 0.05
2 4
Combustible Gases
C H 25 – 335 0.11 0.71 0.06
2 2
Gas C° - Range Detection Repetition
A
CO 45 – 9300 0.22 0.78
n ppm MDL MRL
95 % 95 %
Oxygen and Nitrogen
H 11.3 12.7 1.0
n ppm S (r) S (R) B
n 50 % n 50 % n
CO 6.9 – 13.8 6.9 2.7
O 4630 – 4670 0.24 0.63 0.41 – 1.05
CH 2.2 – 4.3 3.2 1.5
N 27000 – 0.17 0.35 0.49 – 0.04
C H 2.7 – 5.3 2.3 2.1
2 6
C H 2.0 – 4.0 2.4 2.3
2 4
A
C H 1.5 – 3.0 1.8 0.5
See text.
2 2
D
...
Frequently Asked Questions
ASTM D3612-02(2017) is a standard published by ASTM International. Its full title is "Standard Test Method for Analysis of Gases Dissolved in Electrical Insulating Oil by Gas Chromatography". This standard covers: SIGNIFICANCE AND USE 5.1 Oil and oil-immersed electrical insulation materials may decompose under the influence of thermal and electrical stresses, and in doing so, generate gaseous decomposition products of varying composition which dissolve in the oil. The nature and amount of the individual component gases that may be recovered and analyzed may be indicative of the type and degree of the abnormality responsible for the gas generation. The rate of gas generation and changes in concentration of specific gases over time are also used to evaluate the condition of the electric apparatus. Note 1: Guidelines for the interpretation of gas-in-oil data are given in IEEE C57.104. SCOPE 1.1 This test method covers three procedures for extraction and measurement of gases dissolved in electrical insulating oil having a viscosity of 20 cSt (100 SUS) or less at 40°C (104°F), and the identification and determination of the individual component gases extracted. Other methods have been used to perform this analysis. 1.2 The individual component gases that may be identified and determined include: Hydrogen—H2 Oxygen—O2 Nitrogen—N2 Carbon monoxide—CO Carbon dioxide—CO2 Methane—CH4 Ethane—C2H6 Ethylene—C2H4 Acetylene—C2H2 Propane—C3H8 Propylene—C3H6 1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. For specific warning statements see 6.1.8, 30.2.2 and 30.3.1. 1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
SIGNIFICANCE AND USE 5.1 Oil and oil-immersed electrical insulation materials may decompose under the influence of thermal and electrical stresses, and in doing so, generate gaseous decomposition products of varying composition which dissolve in the oil. The nature and amount of the individual component gases that may be recovered and analyzed may be indicative of the type and degree of the abnormality responsible for the gas generation. The rate of gas generation and changes in concentration of specific gases over time are also used to evaluate the condition of the electric apparatus. Note 1: Guidelines for the interpretation of gas-in-oil data are given in IEEE C57.104. SCOPE 1.1 This test method covers three procedures for extraction and measurement of gases dissolved in electrical insulating oil having a viscosity of 20 cSt (100 SUS) or less at 40°C (104°F), and the identification and determination of the individual component gases extracted. Other methods have been used to perform this analysis. 1.2 The individual component gases that may be identified and determined include: Hydrogen—H2 Oxygen—O2 Nitrogen—N2 Carbon monoxide—CO Carbon dioxide—CO2 Methane—CH4 Ethane—C2H6 Ethylene—C2H4 Acetylene—C2H2 Propane—C3H8 Propylene—C3H6 1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. For specific warning statements see 6.1.8, 30.2.2 and 30.3.1. 1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
ASTM D3612-02(2017) is classified under the following ICS (International Classification for Standards) categories: 29.040.10 - Insulating oils. The ICS classification helps identify the subject area and facilitates finding related standards.
ASTM D3612-02(2017) has the following relationships with other standards: It is inter standard links to ASTM D3612-02(2009), ASTM D2140-23, ASTM D2140-23e1, ASTM E260-96(2019), ASTM D2300-08(2017), ASTM E260-96(2011), ASTM D4051-10, ASTM D2300-08, ASTM D2779-92(2007), ASTM D2780-92(2007), ASTM E260-96(2006), ASTM D4051-99(2004), ASTM D2140-03, ASTM E260-96, ASTM E260-96(2001). Understanding these relationships helps ensure you are using the most current and applicable version of the standard.
You can purchase ASTM D3612-02(2017) directly from iTeh Standards. The document is available in PDF format and is delivered instantly after payment. Add the standard to your cart and complete the secure checkout process. iTeh Standards is an authorized distributor of ASTM standards.
The ASTM D3612-02(2017) standard provides a comprehensive test method for the analysis of gases dissolved in electrical insulating oil using gas chromatography. The scope of this standard is significant as it addresses the critical need to monitor the condition of oil and oil-immersed electrical insulation materials, which may decompose due to thermal and electrical stresses, creating gaseous decomposition products. This aspect of the standard is particularly valuable for evaluating the operational integrity and safety of electrical equipment. One of the strengths of ASTM D3612-02(2017) is its detailed approach to identifying and quantifying various gases, such as hydrogen, oxygen, nitrogen, and several hydrocarbons (e.g., methane, ethane, and acetylene). The method encompasses three procedural approaches for the extraction and measurement of these dissolved gases, specifically targeting oils with a viscosity of 20 cSt or less at 40°C. This targeted analysis ensures that users can obtain relevant data for a broad spectrum of electrical insulating oils. Moreover, the standard's ability to correlate individual component gases with specific electrical abnormalities makes it an essential tool for predictive maintenance. Analyzing gas levels over time allows users to identify potential failures before they occur, which can lead to significant cost savings and enhanced safety in electrical operations. The relevance of this standard is further underscored by its alignment with internationally recognized principles for standardization. It facilitates a consistent approach to gas analysis in the electrical insulation sector, contributing to improved safety and performance measures globally. Overall, ASTM D3612-02(2017) is a pivotal document for professionals engaged in monitoring and managing the health of electrical insulation systems.
ASTM D3612-02(2017)은 전기 절연유에 용해된 가스를 가스 크로마토그래피를 통해 분석하는 표준 시험 방법입니다. 이 표준의 주요 범위는 열 및 전기 스트레스의 영향을 받는 오일과 오일 침식 전기 절연재료의 분해에 따른 기체 분해 생성물의 분석을 포함합니다. 이러한 기체 구성 요소는 고장 원인과 정도를 나타내는 중요한 지표로 활용되며, 가스 생성 속도와 특정 기체의 농도 변화는 전기 기계의 상태를 평가하는 데 필수적입니다. 이 표준에서는 20 cSt (100 SUS) 이하의 점도를 가진 전기 절연유에서 용해된 가스를 추출하고 측정하는 세 가지 절차를 다루고 있습니다. 식별 가능한 개별 기체에는 수소(H2), 산소(O2), 질소(N2), 일산화탄소(CO), 이산화탄소(CO2), 메탄(CH4), 에탄(C2H6), 에티렌(C2H4), 아세틸렌(C2H2), 프로판(C3H8), 프로필렌(C3H6) 등이 포함되어 있습니다. 이는 전기 절연유의 품질과 전반적인 상태를 평가하는 데 중요한 역할을 합니다. ASTM D3612-02(2017)은 국제적으로 인정받는 표준화 원칙에 따라 개발되었으며, 그 사용에 있어 안전, 건강, 환경 관행을 수립하는 책임은 사용자에게 있습니다. 따라서 이 표준은 가스 물질에 대한 중요한 이해를 제공함으로써 전기 절연유의 분석과 함께 관련 절차의 실용성을 강조합니다. 이로 인해, 전기 기계 및 장비의 유지보수 및 안전성을 증대시키는 데 필수적인 역할을 수행합니다.
ASTM D3612-02(2017)は、電気絶縁油に溶解しているガスの分析に関する標準試験方法を提供します。この標準の範囲は、温度および電気ストレスの影響下で分解する油や油浸電気絶縁材料から生成されるガスの組成と数量を評価することです。生成されるガスの特性は、異常事象の種類や程度を示す指標となるため、電気装置の状態評価にも重要な役割を果たします。 標準の強みは、その具体的な試験手順にあります。ASTM D3612では、40°Cで粘度20 cSt(100 SUS)以下の電気絶縁油中の溶解ガスの抽出と測定のための三つの手順が明確に規定されています。この標準に従うことで、ユーザーは水素、酸素、窒素、一酸化炭素、二酸化炭素、メタン、エタン、エチレン、アセチレン、プロパン、プロピレンなどの個々の成分ガスを同定し、定量することが可能となります。 また、ASTM D3612は、ガス分析の解釈に関する指針をIEEE C57.104で提供しており、実務者にとって非常に有用です。この標準は、国際的に認識された標準化の原則に従って開発されており、グローバルなコンテクストでも適用可能です。 ユーザーはこの標準を使用する際に、関連する安全性、健康、および環境の実践を確立する責任があり、規制制限の適用可能性を判断することが求められます。このように、ASTM D3612-02(2017)は、電気絶縁油に関するガス分析の信頼性と有効性を保証するための重要な基準であると言えます。







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.
Loading comments...