ASTM D5284-09(2017)
(Test Method)Standard Test Method for Sequential Batch Extraction of Waste with Acidic Extraction Fluid
Standard Test Method for Sequential Batch Extraction of Waste with Acidic Extraction Fluid
SIGNIFICANCE AND USE
4.1 This test method is intended as a means for obtaining sequential extracts of a waste. The extracts may be used to estimate the release of certain constituents of the waste under the laboratory conditions described in this test method.
4.2 The pH of the extraction fluid used in this test method is to reflect the pH of acidic precipitation in the geographic region in which the waste being tested is to be disposed.
Note 1: Possible sources of information concerning the pH of precipitation in the geographic region of interest include state and federal environmental agencies, state universities, libraries, etc.
Note 2: For sequential batch extraction of waste using a nonacidic extraction fluid, see Test Method D4793.
4.3 An intent of this test method is for the final pH of each of the extracts to reflect the interaction of the extractant with the buffering capacity of the waste.
4.4 This test method is not intended to provide extracts that are representative of the actual leachate produced from a waste in the field or to produce extracts to be used as the sole basis of engineering design.
4.5 This test method has not been demonstrated to simulate actual disposal site leaching conditions.
4.6 This test method produces extracts that are amenable to the determination of both major and minor (trace) constituents. When minor constituents are being determined, it is especially important that precautions be taken in sample storage and handling to avoid possible contamination of the samples.
4.7 This test method has been tested to determine its applicability to certain inorganic components in the waste. This test method has not been tested for applicability to organic substances, volatile matter (see Note 5), or biologically active samples.
4.8 The agitation technique, rate, liquid-to-solid ratio, and filtration conditions specified in the procedure may not be suitable for extracting all types of wastes (see Sections 7 and 8 and Appendix X1).
SCOPE
1.1 This test method provides a procedure for the sequential leaching of a waste containing at least 5 % dry solids in order to generate solutions to be used to determine the constituents leached under the specified testing conditions.
1.2 This test method calls for the shaking of a known weight of waste with acidic extraction fluid of a specified composition as well as the separation of the liquid phase for analysis. The pH of the extraction fluid is to reflect the pH of acidic precipitation in the geographic region in which the waste being tested is to be disposed. The procedure is conducted ten times in sequence on the same sample of waste, and it generates ten solutions.
1.3 This test method is intended to describe the procedure for performing sequential batch extractions only. It does not describe all types of sampling and analytical requirements that may be associated with its application.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D5284 − 09 (Reapproved 2017)
Standard Test Method for
Sequential Batch Extraction of Waste with Acidic Extraction
Fluid
This standard is issued under the fixed designation D5284; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 Thistestmethodprovidesaprocedureforthesequential
D75/D75MPractice for Sampling Aggregates
leaching of a waste containing at least 5% dry solids in order
D420GuidetoSiteCharacterizationforEngineeringDesign
to generate solutions to be used to determine the constituents
leached under the specified testing conditions. and Construction Purposes (Withdrawn 2011)
D653Terminology Relating to Soil, Rock, and Contained
1.2 Thistestmethodcallsfortheshakingofaknownweight
Fluids
ofwastewithacidicextractionfluidofaspecifiedcomposition
D1129Terminology Relating to Water
as well as the separation of the liquid phase for analysis. The
D1193Specification for Reagent Water
pH of the extraction fluid is to reflect the pH of acidic
D2234/D2234MPractice for Collection of a Gross Sample
precipitationinthegeographicregioninwhichthewastebeing
of Coal
tested is to be disposed. The procedure is conducted ten times
D2777Practice for Determination of Precision and Bias of
in sequence on the same sample of waste, and it generates ten
Applicable Test Methods of Committee D19 on Water
solutions.
D3370Practices for Sampling Water from Closed Conduits
1.3 This test method is intended to describe the procedure
D4793Test Method for Sequential Batch Extraction of
for performing sequential batch extractions only. It does not
Waste with Water
describe all types of sampling and analytical requirements that
may be associated with its application.
3. Terminology
1.4 The values stated in SI units are to be regarded as 3.1 Definitions—For definitions of terms used in this test
method, see Terminology D1129.
standard. No other units of measurement are included in this
standard.
3.2 Symbols—Variableslistedinthistestmethodaredefined
1.5 This standard does not purport to address all of the in the individual sections in which they are discussed.Alist of
safety concerns, if any, associated with its use. It is the the defined variables is also provided in Section 11.
responsibility of the user of this standard to establish appro-
4. Significance and Use
priate safety, health and environmental practices and deter-
mine the applicability of regulatory limitations prior to use.
4.1 This test method is intended as a means for obtaining
1.6 This international standard was developed in accor-
sequential extracts of a waste. The extracts may be used to
dance with internationally recognized principles on standard-
estimate the release of certain constituents of the waste under
ization established in the Decision on Principles for the
the laboratory conditions described in this test method.
Development of International Standards, Guides and Recom-
4.2 ThepHoftheextractionfluidusedinthistestmethodis
mendations issued by the World Trade Organization Technical
toreflectthepHofacidicprecipitationinthegeographicregion
Barriers to Trade (TBT) Committee.
in which the waste being tested is to be disposed.
1 2
This test method is under the jurisdiction ofASTM Committee D34 on Waste For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Management and is the direct responsibility of Subcommittee D34.01.04 on Waste contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Leaching Techniques. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Sept. 1, 2017. Published September 2017. Originally the ASTM website.
approved in 1992. Last previous edition approved in 2009 as D5284 – 09. DOI: The last approved version of this historical standard is referenced on
10.1520/D5284-09R17. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5284 − 09 (2017)
NOTE1—PossiblesourcesofinformationconcerningthepHofprecipi-
5.13 pH Meter—Any pH meter with a readability of 0.01
tation in the geographic region of interest include state and federal
units and an accuracy of 60.05 units at 25°C.
environmental agencies, state universities, libraries, etc.
5.14 Carboy-Type Container, with spigot, 20- to 50-L
NOTE 2—For sequential batch extraction of waste using a nonacidic
capacity,ofacompositionsuitabletothenatureoftheanalyses
extraction fluid, see Test Method D4793.
to be performed (see Practices D3370).
4.3 An intent of this test method is for the final pH of each
5.15 Large Glass Funnel.
of the extracts to reflect the interaction of the extractant with
5.16 Crucibles, porcelain, 20-mL capacity each, two per
the buffering capacity of the waste.
waste.
4.4 This test method is not intended to provide extracts that
5.17 Analytical Balance, capable of weighing to 0.1 mg.
arerepresentativeoftheactualleachateproducedfromawaste
5.18 Wash Bottle, 500-mL capacity.
in the field or to produce extracts to be used as the sole basis
of engineering design.
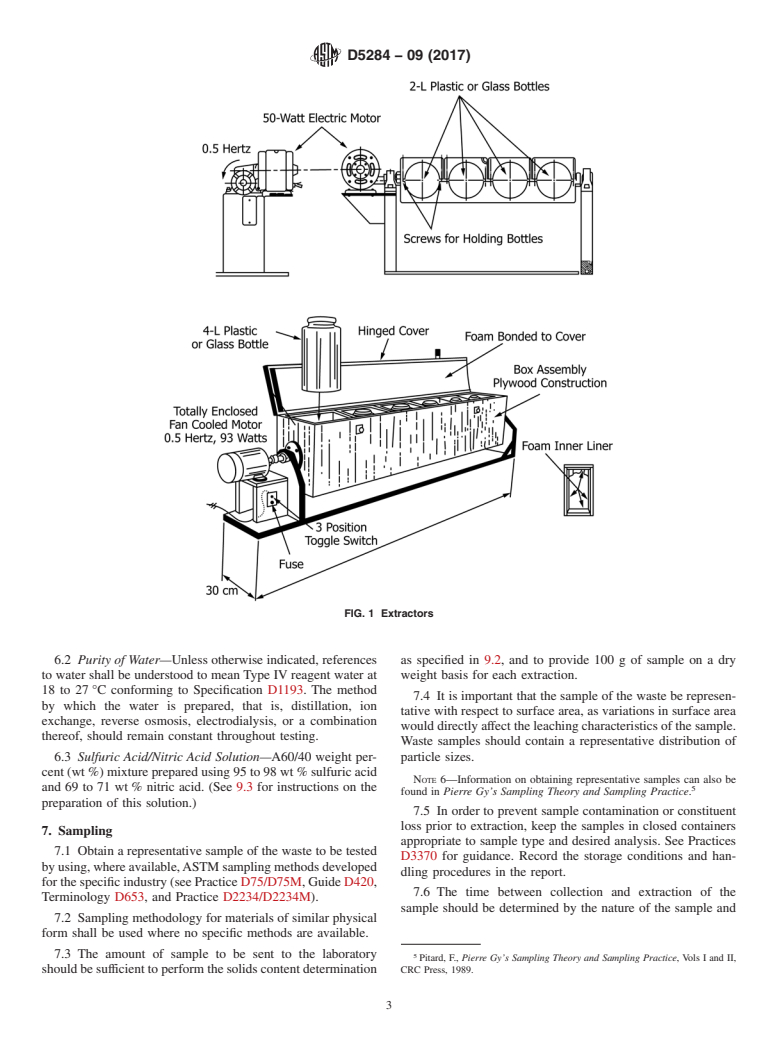
5.19 Agitation Equipment, of any type that rotates the
extraction vessel in an end-over-end fashion at a rate of 0.5 6
4.5 This test method has not been demonstrated to simulate
0.03Hzsuchthattheaxisofrotationishorizontalanditpasses
actual disposal site leaching conditions.
through the center of the bottle (see Fig. 1 and Appendix X1).
4.6 This test method produces extracts that are amenable to
NOTE 3—Similar devices having a different axial arrangement may be
thedeterminationofbothmajorandminor(trace)constituents.
used if equivalency can be demonstrated.
When minor constituents are being determined, it is especially
important that precautions be taken in sample storage and 5.20 Pressure Filtration Assembly—A pressure filtration
handling to avoid possible contamination of the samples. device of a composition suitable to the nature of the analyses
to be performed and equipped with a 0.45 or 0.8-µm pore size
4.7 This test method has been tested to determine its
filter (see Note 8).
applicabilitytocertaininorganiccomponentsinthewaste.This
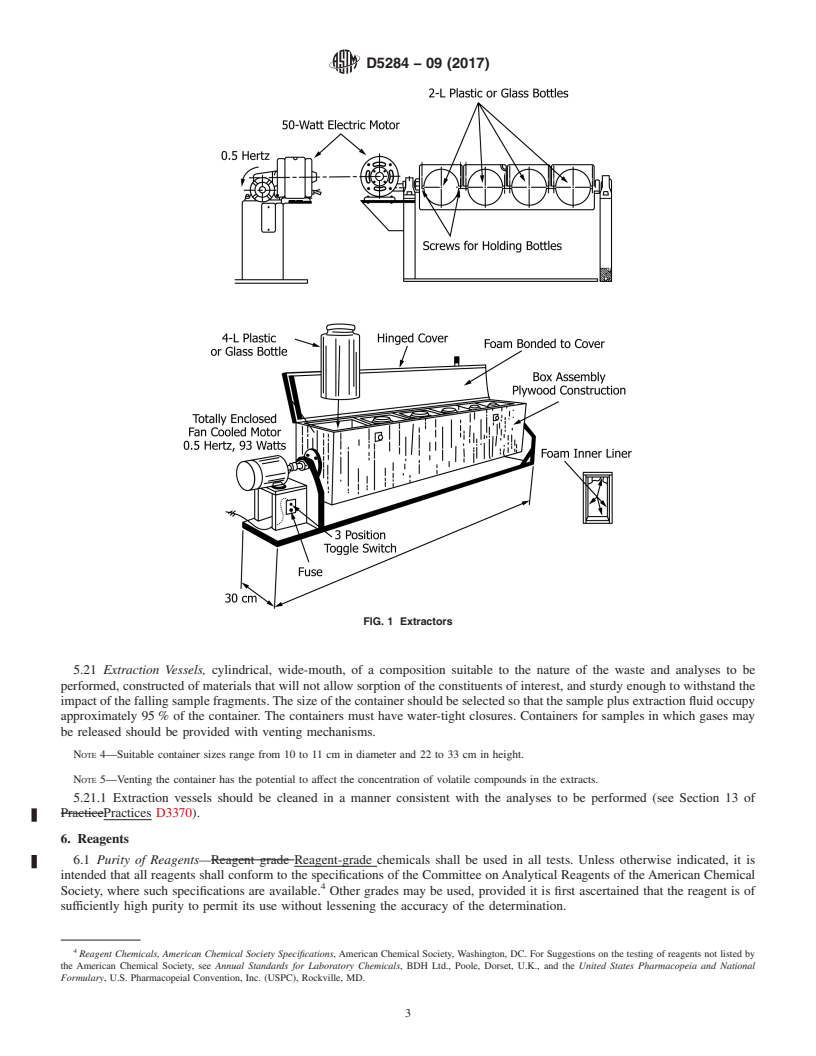
5.21 Extraction Vessels, cylindrical, wide-mouth, of a com-
test method has not been tested for applicability to organic
position suitable to the nature of the waste and analyses to be
substances, volatile matter (see Note 5), or biologically active
performed,constructedofmaterialsthatwillnotallowsorption
samples.
of the constituents of interest, and sturdy enough to withstand
4.8 The agitation technique, rate, liquid-to-solid ratio, and
the impact of the falling sample fragments. The size of the
filtration conditions specified in the procedure may not be
container should be selected so that the sample plus extraction
suitableforextractingalltypesofwastes(seeSections7and8
fluid occupy approximately 95% of the container. The con-
and Appendix X1).
tainers must have water-tight closures. Containers for samples
in which gases may be released should be provided with
5. Apparatus
venting mechanisms.
5.1 Straightedge, such as a thin-edged yardstick.
NOTE 4—Suitable container sizes range from 10 to 11 cm in diameter
and 22 to 33 cm in height.
5.2 Impermeable Sheet, of glazed paper, oil cloth, or other
flexible material of a composition suitable to the analytes of
NOTE 5—Venting the container has the potential to affect the concen-
interest.
tration of volatile compounds in the extracts.
5.3 Drying Pans or Dishes (for example, aluminum tins,
5.21.1 Extraction vessels should be cleaned in a manner
porcelain dishes, glass weighing pans), two per waste, suitable
consistentwiththeanalysestobeperformed(seeSection13of
to the waste being tested and the instructions given in 9.2.
Practices D3370).
5.4 Drying Oven—Any thermostatically controlled drying
6. Reagents
oven capable of maintaining a steady temperature of 62°Cin
a range of 100 to 110°C.
6.1 Purity of Reagents—Reagent-grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
5.5 Desiccator, having a capacity to hold the drying pans
all reagents shall conform to the specifications of the Commit-
described in 5.3 and the crucibles described in 5.16.
tee onAnalytical Reagents of theAmerican Chemical Society,
5.6 Laboratory Balance, capable of weighing to 0.1 g. 4
where such specifications are available. Other grades may be
used, provided it is first ascertained that the reagent is of
5.7 Erlenmeyer Flask, 2-L capacity, equipped with a mag-
sufficiently high purity to permit its use without lessening the
netic stir bar.
accuracy of the determination.
5.8 Magnetic Stir Plate.
5.9 Graduated Cylinder, 1- or 2-L capacity.
Reagent Chemicals, American Chemical Society Specifications, American
5.10 Pipet, 1-mL capacity.
Chemical Society, Washington, DC. For Suggestions on the testing of reagents not
5.11 Volumetric Flask, 1-L capacity.
listed by the American Chemical Society, see Annual Standards for Laboratory
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
5.12 Pipet, 10-mL capacity. (Various other sized pipets,
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
including micropipets, may be necessary for 9.3.2.) MD.
D5284 − 09 (2017)
FIG. 1 Extractors
6.2 Purity of Water—Unless otherwise indicated, references as specified in 9.2, and to provide 100 g of sample on a dry
to water shall be understood to meanType IVreagent water at weight basis for each extraction.
18 to 27°C conforming to Specification D1193. The method
7.4 It is important that the sample of the waste be represen-
by which the water is prepared, that is, distillation, ion
tative with respect to surface area, as variations in surface area
exchange, reverse osmosis, electrodialysis, or a combination
woulddirectlyaffecttheleachingcharacteristicsofthesample.
thereof, should remain constant throughout testing.
Waste samples should contain a representative distribution of
6.3 Sulfuric Acid/Nitric Acid Solution—A60/40 weight per- particle sizes.
cent(wt%)mixturepreparedusing95to98wt%sulfuricacid
NOTE 6—Information on obtaining representative samples can also be
and 69 to 71 wt% nitric acid. (See 9.3 for instructions on the
found in Pierre Gy’s Sampling Theory and Sampling Practice.
preparation of this solution.)
7.5 In order to prevent sample contamination or constituent
loss prior to extraction, keep the samples in closed containers
7. Sampling
appropriate to sample type and desired analysis. See Practices
7.1 Obtain a representative sample of the waste to be tested
D3370 for guidance. Record the storage conditions and han-
byusing,whereavailable,ASTMsamplingmethodsdeveloped
dling procedures in the report.
forthespecificindustry(seePracticeD75/D75M,GuideD420,
7.6 The time between collection and extraction of the
Terminology D653, and Practice D2234/D2234M).
sample should be determined by the nature of the sample and
7.2 Sampling methodology for materials of similar physical
form shall be used where no specific methods are available.
7.3 The amount of sample to be sent to the laboratory
Pitard, F., Pierre Gy’s Sampling Theory and Sampling Practice, Vols I and II,
shouldbesufficienttoperformthesolidscontentdetermination CRC Press, 1989.
D5284 − 09 (2017)
the information desired. See Practices D3370 for guidance. 9.2 Solids Content—Determine the solids content of two
Report the length of time between sample collection and separate portions of the sample as follows:
extraction.
9.2.1 Drytoaconstantweight,at104 62°C,twodishesor
pans of size suitable to the solid waste being tested. Cool in a
8. Sample Preparation
desiccator and weigh. Record the values to 60.1 g.
9.2.2 Place 50 g of the waste to be tested into each pan.
8.1 For free-flowing particulate solid wastes, obtain a
Record the mass of sample in each pan to 60.1 g.
sample of the approximate size required in the test by quarter-
9.2.3 Dry 16 to 20 h at 104 6 2°C. Record the temperature
ing the sample (Section 7) received for testing on an imper-
and time of the drying period.
meable sheet of glazed paper, oil cloth, or other flexible
9.2.4 Cooltoroomtemperatureinadesiccatorandreweigh.
material having a composition suitable to the analytes of
Record the mass to 60.1 g.
interest, as follows:
8.1.1 Empty the sample container into the center of the 9.2.5 Repeatthestepsgivenin9.2.3and9.2.4untilconstant
container-sample masses are obtained. Discard the dried
sheet.
samples following completion of this step.
8.1.2 Gently flatten the sample out with a suitable straight-
9.2.6 Calculate the solids content of the sample from the
edge until it is spread uniformly to a depth at least twice the
maximum particle diameter. data obtained in 9.2.1, 9.2.2, and 9.2.4 as follows:
8.1.3 Remix the sample by lifting a corner of the sheet and
S 5 A/B (1)
drawing it low across to the opposite corner in such a manner
where:
that the material is made to roll over and over and does not
merely slide along. Continue the operation with each corner, A = mass of sample after drying, g,
B = original mass of sample, g, and
proceeding in a clockwise direction. Repeat this operation ten
S = solids content, g/g.
times.
8.1.4 Liftallfourcornersofthesheettowardthecenterand,
Average the two values obtained. Record the solids content.
holding all four corners together, raise the entire sheet into the
9.3 Preparation of Extraction Fluid—Prepare a 60/40 wt%
air to form a pocket for the sample.
mixture of sulfuric acid/nitric acid. Cautiously mix 60 g of
8.1.5 Repeat the procedure described in 8.1.2 to flatten the
concentratedsulfuricacidwith40gofconcentratednitricacid.
sample out.
The preparation of this mixture should be performed in a
8.1.6 Withastraightedge(suchasathin-edgedyardstick)at
laboratory fume hood.
least as long as the flattened mound of sample, gently divide
9.3.1 Using the 60/40 sulfuric acid/nitric acid mixture,
thesampleintoquarters.Makeanefforttoavoidusingpressure
prepare a second solution by diluting 1.0 mL of the 60/40
on the straightedge sufficient to cause damage to the particles.
mixture to 1000 mL using water and a 1-L volumetric flask.
8.1.7 Discard the alternate quarters.
9.3.2 Using the 1/1000 solution prepared in 9.3.1, prepare
8.1.8 If further reduct
...
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D5284 − 09 (Reapproved 2017)
Standard Test Method for
Sequential Batch Extraction of Waste with Acidic Extraction
Fluid
This standard is issued under the fixed designation D5284; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This test method provides a procedure for the sequential 2.1 ASTM Standards:
leaching of a waste containing at least 5 % dry solids in order D75/D75M Practice for Sampling Aggregates
to generate solutions to be used to determine the constituents D420 Guide to Site Characterization for Engineering Design
leached under the specified testing conditions. and Construction Purposes (Withdrawn 2011)
D653 Terminology Relating to Soil, Rock, and Contained
1.2 This test method calls for the shaking of a known weight
Fluids
of waste with acidic extraction fluid of a specified composition
D1129 Terminology Relating to Water
as well as the separation of the liquid phase for analysis. The
D1193 Specification for Reagent Water
pH of the extraction fluid is to reflect the pH of acidic
D2234/D2234M Practice for Collection of a Gross Sample
precipitation in the geographic region in which the waste being
of Coal
tested is to be disposed. The procedure is conducted ten times
D2777 Practice for Determination of Precision and Bias of
in sequence on the same sample of waste, and it generates ten
Applicable Test Methods of Committee D19 on Water
solutions.
D3370 Practices for Sampling Water from Closed Conduits
1.3 This test method is intended to describe the procedure
D4793 Test Method for Sequential Batch Extraction of
for performing sequential batch extractions only. It does not
Waste with Water
describe all types of sampling and analytical requirements that
3. Terminology
may be associated with its application.
1.4 The values stated in SI units are to be regarded as 3.1 Definitions—For definitions of terms used in this test
method, see Terminology D1129.
standard. No other units of measurement are included in this
standard.
3.2 Symbols—Variables listed in this test method are defined
1.5 This standard does not purport to address all of the in the individual sections in which they are discussed. A list of
safety concerns, if any, associated with its use. It is the
the defined variables is also provided in Section 11.
responsibility of the user of this standard to establish appro-
4. Significance and Use
priate safety, health and environmental practices and deter-
mine the applicability of regulatory limitations prior to use. 4.1 This test method is intended as a means for obtaining
1.6 This international standard was developed in accor- sequential extracts of a waste. The extracts may be used to
dance with internationally recognized principles on standard- estimate the release of certain constituents of the waste under
ization established in the Decision on Principles for the the laboratory conditions described in this test method.
Development of International Standards, Guides and Recom-
4.2 The pH of the extraction fluid used in this test method is
mendations issued by the World Trade Organization Technical
to reflect the pH of acidic precipitation in the geographic region
Barriers to Trade (TBT) Committee.
in which the waste being tested is to be disposed.
1 2
This test method is under the jurisdiction of ASTM Committee D34 on Waste For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Management and is the direct responsibility of Subcommittee D34.01.04 on Waste contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Leaching Techniques. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Sept. 1, 2017. Published September 2017. Originally the ASTM website.
approved in 1992. Last previous edition approved in 2009 as D5284 – 09. DOI: The last approved version of this historical standard is referenced on
10.1520/D5284-09R17. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5284 − 09 (2017)
NOTE 1—Possible sources of information concerning the pH of precipi-
5.13 pH Meter—Any pH meter with a readability of 0.01
tation in the geographic region of interest include state and federal
units and an accuracy of 60.05 units at 25 °C.
environmental agencies, state universities, libraries, etc.
5.14 Carboy-Type Container, with spigot, 20- to 50-L
NOTE 2—For sequential batch extraction of waste using a nonacidic capacity, of a composition suitable to the nature of the analyses
extraction fluid, see Test Method D4793.
to be performed (see Practices D3370).
4.3 An intent of this test method is for the final pH of each
5.15 Large Glass Funnel.
of the extracts to reflect the interaction of the extractant with
5.16 Crucibles, porcelain, 20-mL capacity each, two per
the buffering capacity of the waste.
waste.
4.4 This test method is not intended to provide extracts that
5.17 Analytical Balance, capable of weighing to 0.1 mg.
are representative of the actual leachate produced from a waste
5.18 Wash Bottle, 500-mL capacity.
in the field or to produce extracts to be used as the sole basis
of engineering design.
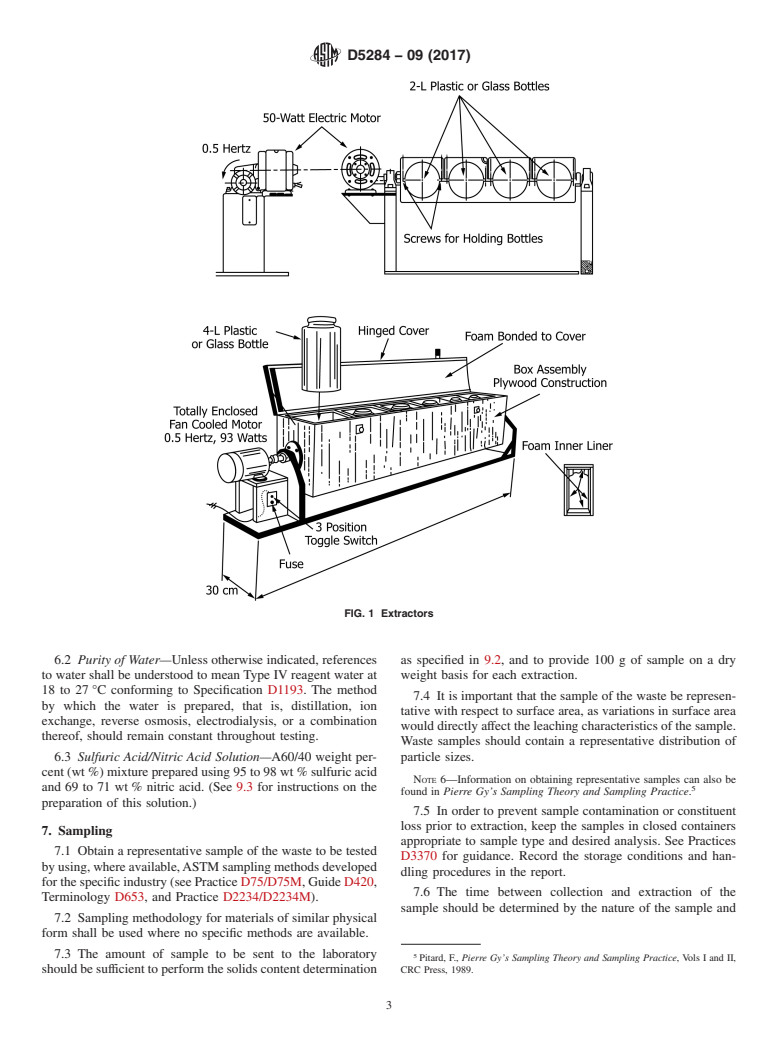
5.19 Agitation Equipment, of any type that rotates the
extraction vessel in an end-over-end fashion at a rate of 0.5 6
4.5 This test method has not been demonstrated to simulate
0.03 Hz such that the axis of rotation is horizontal and it passes
actual disposal site leaching conditions.
through the center of the bottle (see Fig. 1 and Appendix X1).
4.6 This test method produces extracts that are amenable to
NOTE 3—Similar devices having a different axial arrangement may be
the determination of both major and minor (trace) constituents.
used if equivalency can be demonstrated.
When minor constituents are being determined, it is especially
important that precautions be taken in sample storage and 5.20 Pressure Filtration Assembly—A pressure filtration
handling to avoid possible contamination of the samples. device of a composition suitable to the nature of the analyses
to be performed and equipped with a 0.45 or 0.8-µm pore size
4.7 This test method has been tested to determine its
filter (see Note 8).
applicability to certain inorganic components in the waste. This
5.21 Extraction Vessels, cylindrical, wide-mouth, of a com-
test method has not been tested for applicability to organic
position suitable to the nature of the waste and analyses to be
substances, volatile matter (see Note 5), or biologically active
performed, constructed of materials that will not allow sorption
samples.
of the constituents of interest, and sturdy enough to withstand
4.8 The agitation technique, rate, liquid-to-solid ratio, and
the impact of the falling sample fragments. The size of the
filtration conditions specified in the procedure may not be
container should be selected so that the sample plus extraction
suitable for extracting all types of wastes (see Sections 7 and 8
fluid occupy approximately 95 % of the container. The con-
and Appendix X1).
tainers must have water-tight closures. Containers for samples
in which gases may be released should be provided with
5. Apparatus
venting mechanisms.
5.1 Straightedge, such as a thin-edged yardstick.
NOTE 4—Suitable container sizes range from 10 to 11 cm in diameter
and 22 to 33 cm in height.
5.2 Impermeable Sheet, of glazed paper, oil cloth, or other
flexible material of a composition suitable to the analytes of
NOTE 5—Venting the container has the potential to affect the concen-
interest.
tration of volatile compounds in the extracts.
5.3 Drying Pans or Dishes (for example, aluminum tins,
5.21.1 Extraction vessels should be cleaned in a manner
porcelain dishes, glass weighing pans), two per waste, suitable
consistent with the analyses to be performed (see Section 13 of
to the waste being tested and the instructions given in 9.2.
Practices D3370).
5.4 Drying Oven—Any thermostatically controlled drying
6. Reagents
oven capable of maintaining a steady temperature of 62 °C in
a range of 100 to 110 °C.
6.1 Purity of Reagents—Reagent-grade chemicals shall be
used in all tests. Unless otherwise indicated, it is intended that
5.5 Desiccator, having a capacity to hold the drying pans
all reagents shall conform to the specifications of the Commit-
described in 5.3 and the crucibles described in 5.16.
tee on Analytical Reagents of the American Chemical Society,
5.6 Laboratory Balance, capable of weighing to 0.1 g.
where such specifications are available. Other grades may be
used, provided it is first ascertained that the reagent is of
5.7 Erlenmeyer Flask, 2-L capacity, equipped with a mag-
sufficiently high purity to permit its use without lessening the
netic stir bar.
accuracy of the determination.
5.8 Magnetic Stir Plate.
5.9 Graduated Cylinder, 1- or 2-L capacity.
5.10 Pipet, 1-mL capacity. Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For Suggestions on the testing of reagents not
5.11 Volumetric Flask, 1-L capacity.
listed by the American Chemical Society, see Annual Standards for Laboratory
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
5.12 Pipet, 10-mL capacity. (Various other sized pipets,
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
including micropipets, may be necessary for 9.3.2.) MD.
D5284 − 09 (2017)
FIG. 1 Extractors
6.2 Purity of Water—Unless otherwise indicated, references as specified in 9.2, and to provide 100 g of sample on a dry
to water shall be understood to mean Type IV reagent water at weight basis for each extraction.
18 to 27 °C conforming to Specification D1193. The method
7.4 It is important that the sample of the waste be represen-
by which the water is prepared, that is, distillation, ion
tative with respect to surface area, as variations in surface area
exchange, reverse osmosis, electrodialysis, or a combination
would directly affect the leaching characteristics of the sample.
thereof, should remain constant throughout testing.
Waste samples should contain a representative distribution of
6.3 Sulfuric Acid/Nitric Acid Solution—A60/40 weight per- particle sizes.
cent (wt %) mixture prepared using 95 to 98 wt % sulfuric acid
NOTE 6—Information on obtaining representative samples can also be
and 69 to 71 wt % nitric acid. (See 9.3 for instructions on the 5
found in Pierre Gy’s Sampling Theory and Sampling Practice.
preparation of this solution.)
7.5 In order to prevent sample contamination or constituent
loss prior to extraction, keep the samples in closed containers
7. Sampling
appropriate to sample type and desired analysis. See Practices
7.1 Obtain a representative sample of the waste to be tested
D3370 for guidance. Record the storage conditions and han-
by using, where available, ASTM sampling methods developed
dling procedures in the report.
for the specific industry (see Practice D75/D75M, Guide D420,
7.6 The time between collection and extraction of the
Terminology D653, and Practice D2234/D2234M).
sample should be determined by the nature of the sample and
7.2 Sampling methodology for materials of similar physical
form shall be used where no specific methods are available.
7.3 The amount of sample to be sent to the laboratory 5
Pitard, F., Pierre Gy’s Sampling Theory and Sampling Practice, Vols I and II,
should be sufficient to perform the solids content determination CRC Press, 1989.
D5284 − 09 (2017)
the information desired. See Practices D3370 for guidance. 9.2 Solids Content—Determine the solids content of two
Report the length of time between sample collection and separate portions of the sample as follows:
extraction. 9.2.1 Dry to a constant weight, at 104 6 2 °C, two dishes or
pans of size suitable to the solid waste being tested. Cool in a
8. Sample Preparation
desiccator and weigh. Record the values to 60.1 g.
9.2.2 Place 50 g of the waste to be tested into each pan.
8.1 For free-flowing particulate solid wastes, obtain a
Record the mass of sample in each pan to 60.1 g.
sample of the approximate size required in the test by quarter-
9.2.3 Dry 16 to 20 h at 104 6 2 °C. Record the temperature
ing the sample (Section 7) received for testing on an imper-
and time of the drying period.
meable sheet of glazed paper, oil cloth, or other flexible
9.2.4 Cool to room temperature in a desiccator and reweigh.
material having a composition suitable to the analytes of
Record the mass to 60.1 g.
interest, as follows:
9.2.5 Repeat the steps given in 9.2.3 and 9.2.4 until constant
8.1.1 Empty the sample container into the center of the
sheet. container-sample masses are obtained. Discard the dried
samples following completion of this step.
8.1.2 Gently flatten the sample out with a suitable straight-
edge until it is spread uniformly to a depth at least twice the 9.2.6 Calculate the solids content of the sample from the
data obtained in 9.2.1, 9.2.2, and 9.2.4 as follows:
maximum particle diameter.
8.1.3 Remix the sample by lifting a corner of the sheet and
S 5 A/B (1)
drawing it low across to the opposite corner in such a manner
where:
that the material is made to roll over and over and does not
A = mass of sample after drying, g,
merely slide along. Continue the operation with each corner,
B = original mass of sample, g, and
proceeding in a clockwise direction. Repeat this operation ten
S = solids content, g/g.
times.
8.1.4 Lift all four corners of the sheet toward the center and,
Average the two values obtained. Record the solids content.
holding all four corners together, raise the entire sheet into the
9.3 Preparation of Extraction Fluid—Prepare a 60/40 wt %
air to form a pocket for the sample.
mixture of sulfuric acid/nitric acid. Cautiously mix 60 g of
8.1.5 Repeat the procedure described in 8.1.2 to flatten the
concentrated sulfuric acid with 40 g of concentrated nitric acid.
sample out.
The preparation of this mixture should be performed in a
8.1.6 With a straightedge (such as a thin-edged yardstick) at
laboratory fume hood.
least as long as the flattened mound of sample, gently divide
9.3.1 Using the 60/40 sulfuric acid/nitric acid mixture,
the sample into quarters. Make an effort to avoid using pressure
prepare a second solution by diluting 1.0 mL of the 60/40
on the straightedge sufficient to cause damage to the particles.
mixtur
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D5284 − 09 D5284 − 09 (Reapproved 2017)
Standard Test Method for
Sequential Batch Extraction of Waste with Acidic Extraction
Fluid
This standard is issued under the fixed designation D5284; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method provides a procedure for the sequential leaching of a waste containing at least 5 % dry solids in order to
generate solutions to be used to determine the constituents leached under the specified testing conditions.
1.2 This test method calls for the shaking of a known weight of waste with acidic extraction fluid of a specified composition
as well as the separation of the liquid phase for analysis. The pH of the extraction fluid is to reflect the pH of acidic precipitation
in the geographic region in which the waste being tested is to be disposed. The procedure is conducted ten times in sequence on
the same sample of waste, and it generates ten solutions.
1.3 This test method is intended to describe the procedure for performing sequential batch extractions only. It does not describe
all types of sampling and analytical requirements that may be associated with its application.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety problems,concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appropriate safety safety, health and healthenvironmental practices and
determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
D75D75/D75M Practice for Sampling Aggregates
D420 Guide to Site Characterization for Engineering Design and Construction Purposes (Withdrawn 2011)
D653 Terminology Relating to Soil, Rock, and Contained Fluids
D1129 Terminology Relating to Water
D1193 Specification for Reagent Water
D2234/D2234M Practice for Collection of a Gross Sample of Coal
D2777 Practice for Determination of Precision and Bias of Applicable Test Methods of Committee D19 on Water
D3370 Practices for Sampling Water from Closed Conduits
D4793 Test Method for Sequential Batch Extraction of Waste with Water
3. Terminology
3.1 Definitions—For definitions of terms used in this test method, see Terminology D1129.
3.2 Symbols—Variables listed in this test method are defined in the individual sections in which they are discussed. A list of the
defined variables is also provided in Section 11.
This test method is under the jurisdiction of ASTM Committee D34 on Waste Management and is the direct responsibility of Subcommittee D34.01.04 on Waste Leaching
Techniques.
Current edition approved July 1, 2009Sept. 1, 2017. Published October 2009September 2017. Originally approved in 1992. Last previous edition approved in 20042009
e1
as D5284 – 93 (2004)09. . DOI: 10.1520/D5284-09.10.1520/D5284-09R17.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
The last approved version of this historical standard is referenced on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5284 − 09 (2017)
4. Significance and Use
4.1 This test method is intended as a means for obtaining sequential extracts of a waste. The extracts may be used to estimate
the release of certain constituents of the waste under the laboratory conditions described in this test method.
4.2 The pH of the extraction fluid used in this test method is to reflect the pH of acidic precipitation in the geographic region
in which the waste being tested is to be disposed.
NOTE 1—Possible sources of information concerning the pH of precipitation in the geographic region of interest include state and federal environmental
agencies, state universities, libraries, etc.
NOTE 2—For sequential batch extraction of waste using a nonacidic extraction fluid, see Test Method D4793.
4.3 An intent of this test method is for the final pH of each of the extracts to reflect the interaction of the extractant with the
buffering capacity of the waste.
4.4 This test method is not intended to provide extracts that are representative of the actual leachate produced from a waste in
the field or to produce extracts to be used as the sole basis of engineering design.
4.5 This test method has not been demonstrated to simulate actual disposal site leaching conditions.
4.6 This test method produces extracts that are amenable to the determination of both major and minor (trace) constituents.
When minor constituents are being determined, it is especially important that precautions be taken in sample storage and handling
to avoid possible contamination of the samples.
4.7 This test method has been tested to determine its applicability to certain inorganic components in the waste. This test method
has not been tested for applicability to organic substances, volatile matter (see Note 5), or biologically active samples.
4.8 The agitation technique, rate, liquid-to-solid ratio, and filtration conditions specified in the procedure may not be suitable
for extracting all types of wastes (see Sections 7 and 8 and Appendix X1).
5. Apparatus
5.1 Straight Edge, Straightedge, such as a thin-edged yardstick.
5.2 Impermeable Sheet, of glazed paper, oil cloth, or other flexible material of a composition suitable to the analytes of interest.
5.3 Drying Pans or Dishes (for example, aluminum tins, porcelain dishes, glass weighing pans), two per waste, suitable to the
waste being tested and the instructions given in 9.2.
5.4 Drying Oven—Any thermostatically controlled drying oven capable of maintaining a steady temperature of 62°C62 °C in
a range of 100 to 110°C.110 °C.
5.5 Desiccator, having a capacity to hold the drying pans described in 5.3 and the crucibles described in 5.16.
5.6 Laboratory Balance, capable of weighing to 0.1 g.
5.7 Erlenmeyer Flask, 2-L capacity, equipped with a magnetic stir bar.
5.8 Magnetic Stir Plate.
5.9 Graduated Cylinder, 11- or 2-L capacity.
5.10 Pipet, 1-mL capacity.
5.11 Volumetric Flask, 1-L capacity.
5.12 Pipet, 10-mL capacity. (Various other sized pipets, including micropipets, may be necessary for 9.3.2.)
5.13 pH Meter—Any pH meter with a readability of 0.01 units and an accuracy of 60.05 units at 25°C.25 °C.
5.14 Carboy-typeCarboy-Type Container, with spigot, 2020- to 50-L capacity, of a composition suitable to the nature of the
analyses to be performed (see Practices D3370).
5.15 Large Glass Funnel.
5.16 Crucibles, porcelain, 20-mL capacity each, two per waste.
5.17 Analytical Balance, capable of weighing to 0.1 mg.
5.18 Wash Bottle, 500-mL capacity.
5.19 Agitation Equipment, of any type that rotates the extraction vessel in an end-over-end fashion at a rate of 0.5 6 0.03 Hz
such that the axis of rotation is horizontal and it passes through the center of the bottle (see Fig. 1 and Appendix X1).
NOTE 3—Similar devices having a different axial arrangement may be used if equivalency can be demonstrated.
5.20 Pressure Filtration Assembly—A pressure filtration device of a composition suitable to the nature of the analyses to be
performed and equipped with a 0.45 or 0.8-μm pore size filter (see Note 8).
D5284 − 09 (2017)
FIG. 1 Extractors
5.21 Extraction Vessels, cylindrical, wide-mouth, of a composition suitable to the nature of the waste and analyses to be
performed, constructed of materials that will not allow sorption of the constituents of interest, and sturdy enough to withstand the
impact of the falling sample fragments. The size of the container should be selected so that the sample plus extraction fluid occupy
approximately 95 % of the container. The containers must have water-tight closures. Containers for samples in which gases may
be released should be provided with venting mechanisms.
NOTE 4—Suitable container sizes range from 10 to 11 cm in diameter and 22 to 33 cm in height.
NOTE 5—Venting the container has the potential to affect the concentration of volatile compounds in the extracts.
5.21.1 Extraction vessels should be cleaned in a manner consistent with the analyses to be performed (see Section 13 of
PracticePractices D3370).
6. Reagents
6.1 Purity of Reagents—Reagent grade Reagent-grade chemicals shall be used in all tests. Unless otherwise indicated, it is
intended that all reagents shall conform to the specifications of the Committee on Analytical Reagents of the American Chemical
Society, where such specifications are available. Other grades may be used, provided it is first ascertained that the reagent is of
sufficiently high purity to permit its use without lessening the accuracy of the determination.
Reagent Chemicals, American Chemical Society Specifications, American Chemical Society, Washington, DC. For Suggestions on the testing of reagents not listed by
the American Chemical Society, see Annual Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia and National
Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, MD.
D5284 − 09 (2017)
6.2 Purity of Water—Unless otherwise indicated, references to water shall be understood to mean Type IV reagent water at 18
to 27°C27 °C conforming to Specification D1193. The method by which the water is prepared, that is, distillation, ion exchange,
reverse osmosis, electrodialysis, or a combination thereof, should remain constant throughout testing.
6.3 Sulfuric Acid/Nitric Acid Solution—A60/40 weight percent (wt %) mixture prepared using 95 to 98 wt % sulfuric acid and
69 to 71 wt % nitric acid. (See 9.3 for instructions on the preparation of this solution.)
7. Sampling
7.1 Obtain a representative sample of the waste to be tested by using, where available, ASTM sampling methods developed for
the specific industry (see Practice D75D75/D75M, Guide D420, Terminology D653, and Test Method Practice D2234/D2234M).
7.2 Sampling methodology for materials of similar physical form shall be used where no specific methods are available.
7.3 The amount of sample to be sent to the laboratory should be sufficient to perform the solids content determination as
specified in 9.2, and to provide 100 g of sample on a dry weight basis for each extraction.
7.4 It is important that the sample of the waste be representative with respect to surface area, as variations in surface area would
directly affect the leaching characteristics of the sample. Waste samples should contain a representative distribution of particle
sizes.
NOTE 6—Information on obtaining representative samples can also be found in Pierre Gy’s Sampling Theory and Sampling Practice.
7.5 In order to prevent sample contamination or constituent loss prior to extraction, keep the samples in closed containers
appropriate to sample type and desired analysis. See Practices D3370 for guidance. Record the storage conditions and handling
procedures in the report.
7.6 The time between collection and extraction of the sample should be determined by the nature of the sample and the
information desired. See Practices D3370 for guidance. Report the length of time between sample collection and extraction.
8. Sample Preparation
8.1 For free-flowing particulate solid wastes, obtain a sample of the approximate size required in the test by quartering the
sample (Section 7) received for testing on an impermeable sheet of glazed paper, oil cloth, or other flexible material having a
composition suitable to the analytes of interest, as follows:
8.1.1 Empty the sample container into the center of the sheet.
8.1.2 Gently flatten the sample out with a suitable straightedge until it is spread uniformly to a depth at least twice the maximum
particle diameter.
8.1.3 Remix the sample by lifting a corner of the sheet and drawing it low across to the opposite corner in such a manner that
the material is made to roll over and over and does not merely slide along. Continue the operation with each corner, proceeding
in a clockwise direction. Repeat this operation ten times.
8.1.4 Lift all four corners of the sheet toward the center and, holding all four corners together, raise the entire sheet into the air
to form a pocket for the sample.
8.1.5 Repeat the procedure described in 8.1.2 to flatten the sample out.
8.1.6 With a straightedge (such as a thin-edged yardstick) at least as long as the flattened mound of sample, gently divide the
sample into quarters. Make an effort to avoid using pressure on the straightedge sufficient to cause damage to the particles.
8.1.7 Discard the alternate quarters.
8.1.8 If further reduction of the sample size is necessary, repeat the steps given in 8.1.38.1.3 – 8.1.7 through 8.1.7. Use a sample
size to provide 100 g of solid on a dry weight basis for each extraction. Provide additional samples for the determination of solids
content (see 9.2). Use of a sample size other than 100 g of solid on a dry weight basis for extraction is not recommended; however,
if a different sample size is used, report this fact.
NOTE 7—For other acceptable methods of mixing and subsampling free-flowing solid particulate wastes, see Pierre Gy’s Sampling Theory and
Sampling Practice. The method of subsampling should be determined by the physical properties of the waste, analytes of interest, and equipment
available.
8.2 For field-cored solid wastes or castings produced in the laboratory, cut a representative section weighing approximately 100
g for testing, plus samples for the determination of solids content. Shape the sample so that the leaching solution will cover the
material to be leached.
8.3 For multiphasic wastes, mix thoro
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.