ASTM F1541-00
(Specification)Standard Specification and Test Methods for External Skeletal Fixation Devices
Standard Specification and Test Methods for External Skeletal Fixation Devices
SCOPE
1.1 This specification provides a characterization of the design and mechanical function of external skeletal fixation devices (ESFDs), test methods for characterization of ESFD mechanical properties, and identifies needs for further development of test methods and performance criteria. The ultimate goal is to develop a specification, which defines performance criteria and methods for measurement of performance-related mechanical characteristics of ESFDs and their fixation to bone. It is not the intention of this specification to define levels of performance or case-specific clinical performance of the devices, as insufficient knowledge is available to predict the consequences of the use of any of these devices in individual patients for specific activities of daily living. Furthermore, it is not the intention of this specification to describe or specify specific designs for ESFDs.
1.2 This specification describes ESFDs for surgical fixation of the skeletal system. It provides basic ESFD geometrical definitions, dimensions, classification, and terminology; material specifications; performance definitions; test methods; and characteristics determined to be important to the in-vivo performance of the device.
1.3 This specification includes a terminology and classification annex and five standard test method annexes as follows:
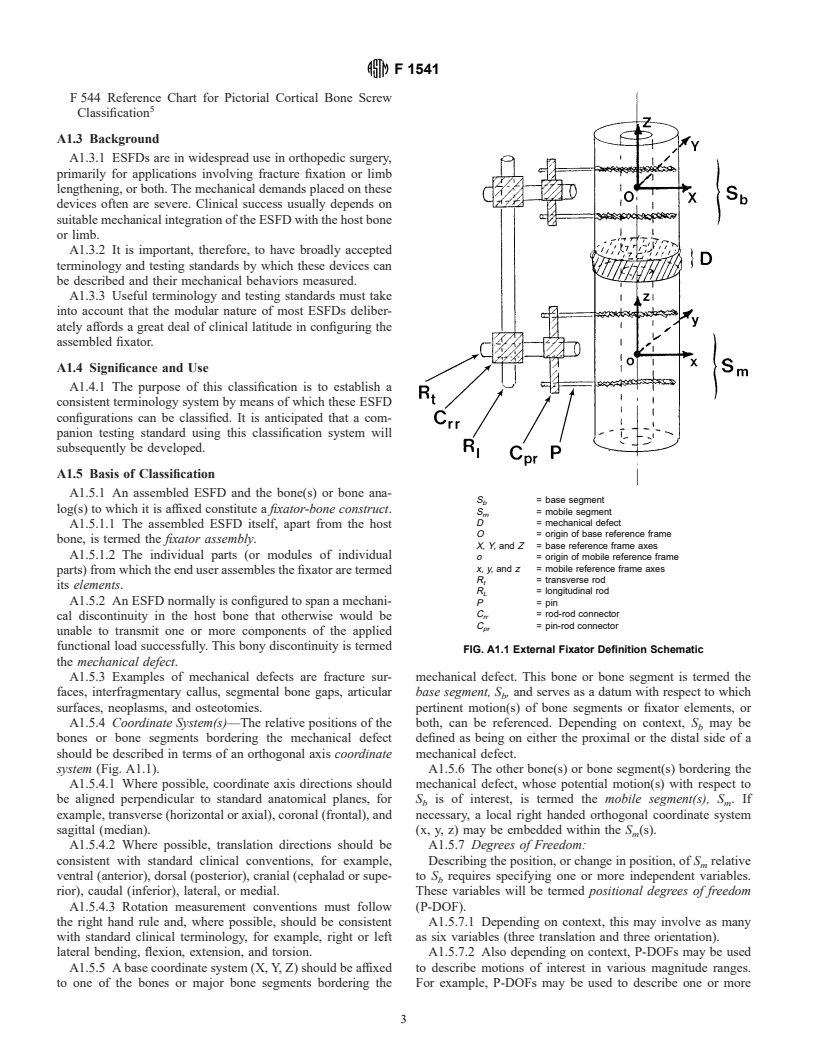
1.3.1 Classification of External Fixators--Annex A1.
1.3.2 Test Method for External Skeletal Fixator Connectors--Annex A2.
1.3.3 Test Method for Determining In-Plane Compressive Properties of Circular Ring or Ring Segment Bridge Elements--Annex A3.
1.3.4 Test Method for External Skeletal Fixator Joints--Annex A4-.
1.3.5 Test Method for External Skeletal Fixator Pin Anchorage Elements--Annex A5.
1.3.6 Test Method for External Skeletal Fixator Subassemblies--Annex A6.
1.3.7 Test Method for External Skeletal Fixator/Constructs Subassemblies--Annex A7.
1.4 A rationale is given in Appendix X1.
1.5 The values stated in SI units are to be regarded as the standard.
1.6 The following safety hazards caveat pertains only to the test method portions (Annex A2-Annex A6):
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 1541 – 00
Standard Specification and Test Methods for
1
External Skeletal Fixation Devices
This standard is issued under the fixed designation F 1541; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 1.5 The values stated in SI units are to be regarded as the
standard.
1.1 This specification provides a characterization of the
1.6 The following safety hazards caveat pertains only to the
design and mechanical function of external skeletal fixation
test method portions (Annex A2-Annex A6):
devices (ESFDs), test methods for characterization of ESFD
1.7 This standard does not purport to address all of the
mechanical properties, and identifies needs for further devel-
safety concerns, if any, associated with its use. It is the
opment of test methods and performance criteria. The ultimate
responsibility of the user of this standard to establish appro-
goal is to develop a specification, which defines performance
priate safety and health practices and determine the applica-
criteria and methods for measurement of performance-related
bility of regulatory limitations prior to use.
mechanical characteristics of ESFDs and their fixation to bone.
It is not the intention of this specification to define levels of
2. Referenced Documents
performance or case-specific clinical performance of the de-
2.1 ASTM Standards:
vices, as insufficient knowledge is available to predict the
2
A 938 Test Method for Torsion Testing of Wire
consequences of the use of any of these devices in individual
D 790 Test Methods for Flexural Properties of Unreinforced
patients for specific activities of daily living. Furthermore, it is
and Reinforced Plastics and Electrical Insulating Materi-
not the intention of this specification to describe or specify
3
als
specific designs for ESFDs.
4
E 4 Practices for Force Verification of Testing Machines
1.2 This specification describes ESFDs for surgical fixation
F 67 Specification for Unalloyed Titanium for Surgical
of the skeletal system. It provides basic ESFD geometrical
5
Implant Applications
definitions, dimensions, classification, and terminology; mate-
F 90 Specification for Wrought Cobalt-20 Chromium-15
rial specifications; performance definitions; test methods; and
Tungsten-10 Nickel Alloy for Surgical Implant Applica-
characteristics determined to be important to the in-vivo
5
tions (R30605)
performance of the device.
F 136 Specification for Wrought Titanium-6 Aluminum-4
1.3 This specification includes a terminology and classifi-
Vanadium ELI (Extra Low Interstitial) Alloy (UNS
cation annex and five standard test method annexes as follows:
R56401) for Surgical Implant Applications
1.3.1 Classification of External Fixators—Annex A1.
F 138 Specification for Wrought-18 Chromium-14 Nickel-
1.3.2 Test Method for External Skeletal Fixator
2.5 Molybdenum Stainless Steel Bar and Wire for Surgical
Connectors—Annex A2.
5
Implants (UNS S31673)
1.3.3 Test Method for Determining In-Plane Compressive
5
F 366 Specification for Fixation Pins and Wires
Properties of Circular Ring or Ring Segment Bridge
5
F 543 Specification for Metallic Medical Bone Screws
Elements—Annex A3.
F 544 Reference Chart for Pictorial Cortical Bone Screw
1.3.4 Test Method for External Skeletal Fixator Joints—
5
Classification
Annex A4.
F 1058 Specification for Wrought Cobalt-Chromium-Nickel
1.3.5 Test Method for External Skeletal Fixator Pin Anchor-
Molybdenum-Iron Alloys for Surgical Implant Applica-
age Elements—Annex A5.
5
tions (UNS R3003 and UNS R3008)
1.3.6 Test Method for External Skeletal Fixator
F 1264 Specification and Test Methods for Intermedullary
Subassemblies—Annex A6.
5
Fixation Devices
1.3.7 Test Method for External Skeletal Fixator/Constructs
F 1472 Specification for Wrought Titanium-16 Aluminum-4
Subassemblies—Annex A7.
Vanadium Alloy for Surgical Implant Applications (UNS
1.4 A rationale is given in Appendix X1.
5
R56400)
F 1713 Specification for Wrought Titanium-13 Niobium-13
1
This specification is under the jurisdiction of ASTM Committee F04 on
2
Medical and Surgical Materials and Devices and is the direct responsibility of Annual Book of ASTM Standards, Vol 01.03.
3
Subcommittee F04.21 on Osteosynthesis. Annual Book of ASTM Standards, Vol 08.01.
4
Current edition approved Nov. 10, 2000. Published March 2001. Originally Annual Book of ASTM Standards, Vol 03.01.
5
published as F 1541 – 99. Last previous edition F 1541 – 94. Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
F
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.