ASTM F1541-99
(Specification)Standard Specification and Test Methods for External Skeletal Fixation Devices
Standard Specification and Test Methods for External Skeletal Fixation Devices
SCOPE
1.1 This specification provides a characterization of the design and mechanical function of external skeletal fixation devices (ESFDs), test methods for characterization of ESFD mechanical properties, and identifies needs for further development of test methods and performance criteria. The ultimate goal is to develop a specification, which defines performance criteria and methods for measurement of performance-related mechanical characteristics of ESFDs and their fixation to bone. It is not the intention of this specification to define levels of performance or case-specific clinical performance of the devices, as insufficient knowledge is available to predict the consequences of the use of any of these devices in individual patients for specific activities of daily living. Furthermore, it is not the intention of this specification to describe or specify specific designs for ESFDs.
1.2 This specification describes ESFDs for surgical fixation of the skeletal system. It provides basic ESFD geometrical definitions, dimensions, classification, and terminology; material specifications; performance definitions; test methods; and characteristics determined to be important to the in-vivo performance of the device.
1.3 This specification includes a terminology and classification annex and five standard test method annexes as follows:
1.3.1 Classification of External Fixators--Annex A1.
1.3.2 Test Method for External Skeletal Fixator Connectors--Annex A2.
1.3.3 Test Method for Determining In-Plane Compressive Properties of Circular Ring or Ring Segment Bridge Elements--Annex A3.
1.3.4 Test Method for External Skeletal Fixator Joints--Annex A4-.
1.3.5 Test Method for External Skeletal Fixator Pin Anchorage Elements--Annex A5.
1.3.6 Test Method for External Skeletal Fixator Subassemblies--Annex A6.
1.3.7 Test Method for External Skeletal Fixator/Constructs Subassemblies--Annex A7.
1.4 A rationale is given in Appendix X1.
1.5 The values stated in SI units are to be regarded as the standard.
1.6 The following safety hazards caveat pertains only to the test method portions (Annex A2-Annex A6):
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 1541 – 99
Standard Specification and Test Methods for
External Skeletal Fixation Devices
This standard is issued under the fixed designation F 1541; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope test method portions (Annex A2-Annex A6):
1.7 This standard does not purport to address all of the
1.1 This specification provides a characterization of the
safety concerns, if any, associated with its use. It is the
design and mechanical function of external skeletal fixation
responsibility of the user of this standard to establish appro-
devices (ESFDs), test methods for characterization of ESFD
priate safety and health practices and determine the applica-
mechanical properties, and identifies needs for further devel-
bility of regulatory limitations prior to use.
opment of test methods and performance criteria. The ultimate
goal is to develop a specification, which defines performance
2. Referenced Documents
criteria and methods for measurement of performance-related
2.1 ASTM Standards:
mechanical characteristics of ESFDs and their fixation to bone.
A 938 Method for Torsion Testing of Wire
It is not the intention of this specification to define levels of
D 790 Test Methods for Flexural Properties of Unreinforced
performance or case-specific clinical performance of the de-
and Reinforced Plastics and Electrical Insulating Materi-
vices, as insufficient knowledge is available to predict the
als
consequences of the use of any of these devices in individual
E 4 Practices for Force Verification of Testing Machines
patients for specific activities of daily living. Furthermore, it is
F 67 Specification for Unalloyed Titanium for Surgical
not the intention of this specification to describe or specify
Implant Applications
specific designs for ESFDs.
F 90 Specification for Wrought Cobalt-Chromium-
1.2 This specification describes ESFDs for surgical fixation
Tungsten Nickel Alloy for Surgical Implant Applications
of the skeletal system. It provides basic ESFD geometrical
F 136 Specification for Wrought Titanium-6 Al
definitions, dimensions, classification, and terminology; mate-
(Aluminum-4 Vanadium ELI (Extra Low Interstitial) Alloy
rial specifications; performance definitions; test methods; and
(R56401) for Surgical Implant Applications
characteristics determined to be important to the in-vivo
F 138 Specification for Wrought-18 Chromium-14 Nickel-
performance of the device.
2.5 Molybdenum Stainless Steel Bar and Wire for Surgical
1.3 This specification includes a terminology and classifi-
Implants (UNS S31673)
cation annex and five standard test method annexes as follows:
F 366 Specification for Fixation Pins and Wires
1.3.1 Classification of External Fixators—Annex A1.
F 543 Specification for Cortical Bone Screws
1.3.2 Test Method for External Skeletal Fixator
F 544 Reference Chart for Pictorial Cortical Bone Screw
Connectors—Annex A2.
Classification
1.3.3 Test Method for Determining In-Plane Compressive
F 1058 Specification for Wrought Cobalt-Chromium-Nickel
Properties of Circular Ring or Ring Segment Bridge
Molybdenum-Iron Alloys for Surgical Implant Applica-
Elements—Annex A3.
tions (UNS R3003 and UNS R3008)
1.3.4 Test Method for External Skeletal Fixator Joints—
F 1264 Specification and Test Methods for Intermedullary
Annex A4.
Fixation Devices
1.3.5 Test Method for External Skeletal Fixator Pin Anchor-
F 1472 Specification for Wrought Ti-6Al-4V Alloy for Sur-
age Elements—Annex A5.
gical Implant Applications
1.3.6 Test Method for External Skeletal Fixator
F 1713 Specification for Wrought Titanium-13 Niobium-13
Subassemblies—Annex A6.
Zirconium Alloy for Surgical Implant Applications
1.4 A rationale is given in Appendix X1.
1.5 The values stated in SI units are to be regarded as the
3. Terminology
standard.
3.1 Definitions—The definitions of terms relating to exter-
1.6 The following safety hazards caveat pertains only to the
nal fixators are described in Annex A1.
This specification is under the jurisdiction of ASTM Committee F-4 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee Annual Book of ASTM Standards, Vol 01.03.
F04.21 on Osteosynthesis. Annual Book of ASTM Standards, Vol 08.01.
Current edition approved June 10, 1999. Published September 1999. Originally Annual Book of ASTM Standards, Vol 03.01.
published as F 1541 – 94. Last previous edition F 1541 – 94. Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
F 1541
4. Classification wires or thin pins, which pass transverse to the bone long axis,
and which are tensioned deliberately in order to control the
4.1 External skeletal fixators are modular devices assembled
longitudinal stiffness of the fixator. Tensioning these wires or
from component elements.
pins causes appreciable compressive load in the plane of the
4.2 Test methods can address individual elements, for ex-
ring element. A test method for evaluating the mechanical
ample, anchorage elements, bridge elements; subassemblies of
performance of ESFD ring elements in this loading mode is
elements, for example, connectors, joints, ring elements; or, the
described in Annex A3.
entire fixator.
6.2.3 The high loads often developed at ESFD junction sites
4.3 Tests of an entire assembled fixator may include the
fixator alone, or alternatively, the fixator as anchored to a are of concern both because of potentially excessive elastic
representation of the bone(s) upon which it typically would be deformation and because of potential irrecoverable deforma-
mounted in clinical usage. tion. In addition to the connecting element itself (Annex A2),
overall performance of the junction also depends on the
5. Materials
interface between the connecting element and the anchorage,
5.1 All ESFDs made of materials, which have an ASTM
or bridge elements, or both, which it grips. A test method for
standard, shall meet those requirements given in ASTM Stan- evaluating the overall strength, or stiffness, or both, at an
dards listed in 2.1
external fixator joint, as defined in Annex A1 as the connecting
element itself plus its interface with the anchorage, or bridge,
6. Performance Considerations and Test Methods
or both, elements, which it grips, is described in Annex A4.
6.1 Individual Components—The anchorage pins through
6.2.4 The modular nature of many ESFD systems affords
which an ESFD is attached to a skeletal member or members
the surgeon particularly great latitude as to configuration of the
typically experience high flexural, or torsional loads, or both.
frame subassembly, as defined in Annex A1 as the bridge
Often, the majority of the overall compliance of an ESFD is in
elements plus the connecting elements used to join bridge
its anchorage elements. A test method for evaluating the
elements, but specifically excluding the anchorage elements.
mechanical performance of an ESFD anchorage element in
Since configuration of the frame subassembly is a major
either of these loading modes is described in Annex A5.
determinant of overall ESFD mechanical behavior, it is impor-
6.2 Subassemblies of Elements:
tant to have procedures for unambiguously characterizing
6.2.1 The sites of junction between ESFD anchorage ele-
frame subassemblies, both geometrically and mechanically.
ments, for example, pins, and bridge elements, for example,
Test methodology suitable for that purpose is described in
rods, normally require specialized clamping or gripping mem-
Annex A6.
bers, known as connecting elements. Often, connecting ele-
6.3 Entire Assembled Fixator—No test methods are yet
ments are subjected to high loads, especially moments, so
approved for entire assembled fixators.
adequacy of their intrinsic mechanical stiffness, or strength, or
both, is critical to overall fixator performance. A test method
7. Keywords
for evaluating the mechanical performance of ESFD connector
elements is described in Annex A2. 7.1 anchorage element; bending; bridge element; connector;
6.2.2 ESFDs involving ring-type bridge elements are used external skeletal fixation device; fracture fixation; joints;
modularity; orthopaedic medical device; osteosynthesis; sub-
widely both for fracture treatment and for distraction osteo-
genesis. The anchorage elements in such fixators usually are assembly (frame); ring element; terminology; torsion
ANNEXES
(Mandatory Information)
A1. CLASSIFICATION OF EXTERNAL SKELETAL FIXATORS
A1.1 Scope priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
A1.1.1 This classification covers the definitions of basic
A1.2 Referenced Documents
terms and considerations for external skeletal fixation devices
(ESFDs) and the mechanical analyses thereof.
A1.2.1 ASTM Standards:
F 366 Specification for Fixation Pins and Wires
A1.1.2 It is not the intent of this classification to define
F 543 Specification for Cortical Bone Screws
levels of acceptable performance or to make recommendations
F 544 Reference Chart for Pictorial Cortical Bone Screw
concerning the appropriate or preferred clinical usage of these
Classification
devices.
A1.3 Background
A1.1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
A1.3.1 ESFDs are in widespread use in orthopaedic surgery,
responsibility of the user of this standard to establish appro- primarily for applications involving fracture fixation or limb
F 1541
lengthening, or both. The mechanical demands placed on these
devices often are severe. Clinical success usually depends on
suitable mechanical integration of the ESFD with the host bone
or limb.
A1.3.2 It is important, therefore, to have broadly accepted
terminology and testing standards by which these devices can
be described and their mechanical behaviors measured.
A1.3.3 Useful terminology and testing standards must take
into account that the modular nature of most ESFDs deliber-
ately affords a great deal of clinical latitude in configuring the
assembled fixator.
A1.4 Significance and Use
A1.4.1 The purpose of this classification is to establish a
consistent terminology system by means of which these ESFD
configurations can be classified. It is anticipated that a com-
panion testing standard using this classification system will
subsequently be developed.
A1.5 Basis of Classification
A1.5.1 An assembled ESFD and the bone(s) or bone ana-
log(s) to which it is affixed constitute a fixator-bone construct.
A1.5.1.1 The assembled ESFD itself, apart from the host
bone, is termed the fixator assembly.
A1.5.1.2 The individual parts (or modules of individual
parts) from which the end user assembles the fixator are termed
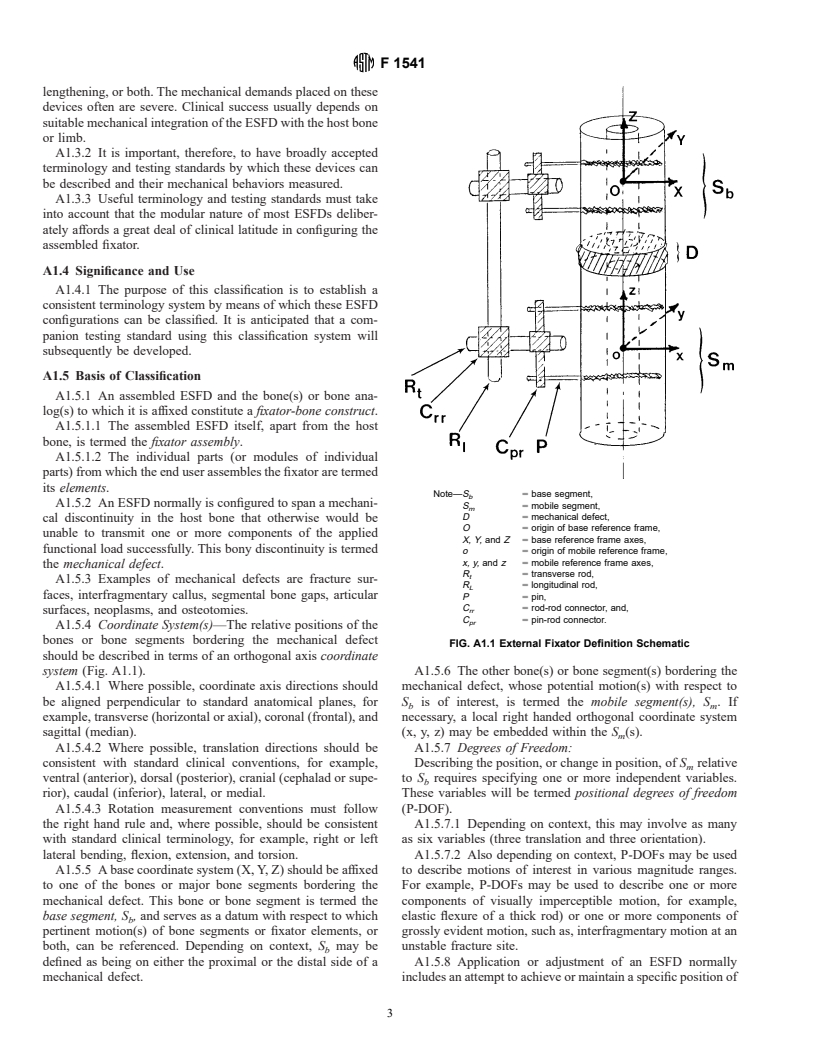
its elements.
Note—S 5 base segment,
b
A1.5.2 An ESFD normally is configured to span a mechani-
S 5 mobile segment,
m
D 5 mechanical defect,
cal discontinuity in the host bone that otherwise would be
O 5 origin of base reference frame,
unable to transmit one or more components of the applied
X, Y, and Z 5 base reference frame axes,
functional load successfully. This bony discontinuity is termed
o 5 origin of mobile reference frame,
x, y, and z 5 mobile reference frame axes,
the mechanical defect.
R 5 transverse rod,
t
A1.5.3 Examples of mechanical defects are fracture sur-
R 5 longitudinal rod,
L
faces, interfragmentary callus, segmental bone gaps, articular
P 5 pin,
C 5 rod-rod connector, and,
rr
surfaces, neoplasms, and osteotomies.
C 5 pin-rod connector.
pr
A1.5.4 Coordinate System(s)—The relative positions of the
bones or bone segments bordering the mechanical defect
FIG. A1.1 External Fixator Definition Schematic
should be described in terms of an orthogonal axis coordinate
system (Fig. A1.1). A1.5.6 The other bone(s) or bone segment(s) bordering the
A1.5.4.1 Where possible, coordinate axis directions should mechanical defect, whose potential motion(s) with respect to
be aligned perpendicular to standard anatomical planes, for S is of interest, is termed the mobile segment(s), S .If
b m
example, transverse (horizontal or axial), coronal (frontal), and necessary, a local right handed orthogonal coordinate system
sagittal (median). (x, y, z) may be embedded within the S (s).
m
A1.5.4.2 Where possible, translation directions should be A1.5.7 Degrees of Freedom:
consistent with standard clinical conventions, for example, Describing the position, or change in position, of S relative
m
ventral (anterior), dorsal (posterior), cranial (cephalad or supe- to S requires specifying one or more independent variables.
b
rior), caudal (inferior), lateral, or medial. These variables will be termed positional degrees of freedom
A1.5.4.3 Rotation measurement conventions must follow (P-DOF).
the right hand rule and, where possible, should be consistent A1.5.7.1 Depending on context, this may involve as many
with standard clinical terminology, for example, right or left as six variables (three translation and three orientation).
A1.5.7.2 Also depending on context, P-DOFs may be used
lateral bending, flexion, extension, and torsion.
A1.5.5 A base coordinate system (X, Y, Z) should be affixed to describe motions of interest in various magnitude ranges.
to one of the bones or major bone segments bordering the For example, P-DOFs may be used to describe one or more
mechanical defect. This bone or bone segment is termed the components of visually imperceptible motion, for example,
base segment, S , and serves as a datum with respect to which elastic flexure of a thick rod) or one or more components of
b
pertinent motion(s) of bone segments or fixator elements, or grossly evident motion, such as, interfragmentary motion at an
both, can be referenced. Depending on context, S may be unstable fracture site.
b
defined as being on either the proximal or the distal side of a A1.5.8 Application or adjustment of an ESFD normally
mechanical defect. includes an attempt to achieve or maintain a specific position of
F 1541
S relative to S . The adjustability afforded by the ESFD in order to engage the attachment. Connectors should be
m b
design for this purpose, most commonly, fracture fragment described in terms of the types of elements that they connect
reduction, will be characterized in terms of adjustment degrees and, where appropriate, in terms of their adjustment or un-
of freedom (A-DOF). locked degrees of freedom. Examples of connectors are pin(-
rod) clamps,
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.