ASTM D8045-17(2023)
(Test Method)Standard Test Method for Acid Number of Crude Oils and Petroleum Products by Catalytic Thermometric Titration
Standard Test Method for Acid Number of Crude Oils and Petroleum Products by Catalytic Thermometric Titration
SIGNIFICANCE AND USE
5.1 Crude oils and oil sands bitumen contain naturally occurring acidic species. Acidity of crude oil has been implicated in corrosion of distribution and process systems. The relative amount of these materials can be determined by titrating with bases. The acid number is a measure of this amount of acidic substance in the oil under the conditions of the test.
5.2 Acid number of crude and distilled petroleum fractions has been measured by Test Method D664. Test Method D664 was developed for the analysis of lubricants and biodiesel. The titration solvent used in Test Method D664 does not properly address dissolving difficult samples such as crude oil, bitumen, and high wax samples addressed in this test method. Refer to Appendix X1.
5.3 Test Method D974 is also not applicable to measuring acidity of crudes and highly colored samples because the indicator is not visible or it is difficult to discern a color change to detect the end point of the titration.
SCOPE
1.1 This test method covers the determination of acidic components in crude oil and petroleum products including waxes, bitumen, base stocks, and asphalts that are soluble in mixtures of xylenes and propan-2-ol. It is applicable for the determination of acids whose dissociation constants in water are larger than 10–9; extremely weak acids whose dissociation constants are smaller than 10–9 do not interfere. The values obtained by this test method may not be numerically equivalent to other acid value measurements. The range of KOH acid numbers included in the precision statement is 0.1 mg/g to 16 mg/g.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. Some specific hazards statements are given in Section 7 on Safety Precautions.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D8045 − 17 (Reapproved 2023)
Standard Test Method for
Acid Number of Crude Oils and Petroleum Products by
Catalytic Thermometric Titration
This standard is issued under the fixed designation D8045; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope by Potentiometric Titration
D974 Test Method for Acid and Base Number by Color-
1.1 This test method covers the determination of acidic
Indicator Titration
components in crude oil and petroleum products including
D1193 Specification for Reagent Water
waxes, bitumen, base stocks, and asphalts that are soluble in
D4057 Practice for Manual Sampling of Petroleum and
mixtures of xylenes and propan-2-ol. It is applicable for the
Petroleum Products
determination of acids whose dissociation constants in water
–9 D4175 Terminology Relating to Petroleum Products, Liquid
are larger than 10 ; extremely weak acids whose dissociation
–9 Fuels, and Lubricants
constants are smaller than 10 do not interfere. The values
D4177 Practice for Automatic Sampling of Petroleum and
obtained by this test method may not be numerically equivalent
Petroleum Products
to other acid value measurements. The range of KOH acid
D5854 Practice for Mixing and Handling of Liquid Samples
numbers included in the precision statement is 0.1 mg ⁄g to
of Petroleum and Petroleum Products
16 mg ⁄g.
D6299 Practice for Applying Statistical Quality Assurance
1.2 The values stated in SI units are to be regarded as
and Control Charting Techniques to Evaluate Analytical
standard. No other units of measurement are included in this
Measurement System Performance
standard.
3. Terminology
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the 3.1 For general terminology, refer to Terminology D4175.
responsibility of the user of this standard to establish appro-
3.2 Definitions:
priate safety, health, and environmental practices and deter-
3.2.1 acid number, n—the quantity of a specified base,
mine the applicability of regulatory limitations prior to use.
expressed in milligrams of potassium hydroxide per gram of
Some specific hazards statements are given in Section 7 on
sample, required to titrate a sample in a specified solvent to a
Safety Precautions.
specified endpoint using a specified detection system.
1.4 This international standard was developed in accor-
3.2.2 catalytic thermometric titration, n—a method to de-
dance with internationally recognized principles on standard-
termine the end point of a chemical reaction through the use a
ization established in the Decision on Principles for the
temperature measuring device and the addition of a chemical to
Development of International Standards, Guides and Recom-
enhance the detection of the endpoint.
mendations issued by the World Trade Organization Technical
3.2.3 crude oil, n—a naturally occurring hydrocarbon
Barriers to Trade (TBT) Committee.
mixture, generally in a liquid state, which may also include
2. Referenced Documents
compounds of sulfur, nitrogen, oxygen, metals, and other
elements.
2.1 ASTM Standards:
D664 Test Method for Acid Number of Petroleum Products
4. Summary of Test Method
4.1 The sample and a fixed mass of paraformaldehyde are
This test method is under the jurisdiction of ASTM Committee D02 on
dissolved in a mixture of xylenes and propan-2-ol. The mixture
Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of
is then titrated with potassium hydroxide using a constant rate
Subcommittee D02.06 on Analysis of Liquid Fuels and Lubricants.
of titrant addition. A plot of the temperature of the reaction
Current edition approved March 1, 2023. Published March 2023. Originally
ɛ1
mixture versus the volume of titrant is generated. An exother-
approved in 2016. Last previous edition approved in 2017 as D8045 – 17 . DOI:
10.1520/D8045-17R23.
mic reaction between the titrant and sample occurs simultane-
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
ously with the endothermic depolymerization of paraformalde-
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
hyde. After all of the acidic material in the sample has reacted,
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. the slope of the plot changes due to the absence of the
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D8045 − 17 (2023)
competitive exothermic acid-base reaction. The change in 8.3 Analytical Balance, capable of weighing to 60.1 mg.
slope is the inflection point. The depolymerization of
8.4 Burette, typically less than 20 mL capacity with a guard
paraformaldehyde is catalytically initiated and does not con-
tube containing adsorbent to retard the rate of titrant reaction
sume a significant quantity of potassium hydroxide. The net
with atmospheric carbon dioxide.
change (positive or negative) in temperature prior to the
8.5 Constant or Variable Speed Propeller Stirrer, sufficient
consumption of the acidic sample will be dependent upon the
to ensure adequate mixing of the sample and titrant.
relative magnitude of the heats of reaction and environmental
influences. 8.6 Titration Beaker, with sufficient volume and made of a
material that does not interact with the sample, titrant or
5. Significance and Use
titration solvent.
5.1 Crude oils and oil sands bitumen contain naturally
8.7 Volumetric Dispenser, capable of consistently delivering
occurring acidic species. Acidity of crude oil has been impli-
desired volume of titration solvent.
cated in corrosion of distribution and process systems. The
relative amount of these materials can be determined by
9. Reagents
titrating with bases. The acid number is a measure of this
9.1 Potassium Hydroxide Solution, Standard Alcoholic,
amount of acidic substance in the oil under the conditions of
(0.1 M)—Add 6 g of potassium hydroxide (KOH) to approxi-
the test.
mately 1 L of propan-2-ol. Boil gently for 10 min to dissolve.
5.2 Acid number of crude and distilled petroleum fractions
Allow the solution to stand for two days and then filter the
has been measured by Test Method D664. Test Method D664
supernatant liquid through a fine sintered-glass funnel. Store
was developed for the analysis of lubricants and biodiesel. The
the solution in a chemically resistant bottle. Dispense in a
titration solvent used in Test Method D664 does not properly
manner such that the solution is protected from atmospheric
address dissolving difficult samples such as crude oil, bitumen,
carbon dioxide (CO ) by means of a guard tube containing
and high wax samples addressed in this test method. Refer to
soda lime or soda non-fibrous silicate absorbents and such that
Appendix X1.
it does not come into contact with cork, rubber, or saponifiable
stopcock grease. Commercially prepared reagent is also suit-
5.3 Test Method D974 is also not applicable to measuring
able. Standardize frequently enough to detect concentration
acidity of crudes and highly colored samples because the
changes of 0.0005 mol ⁄L by standard industry practices or by
indicator is not visible or it is difficult to discern a color change
thermometric titration of known quantities of benzoic acid
to detect the end point of the titration.
solution dissolved in n-heptane. See Appendix for details.
6. Interferences
9.2 Propan-2-ol (anhydrous, also referred to as isopropyl
6.1 Any material that reacts with potassium hydroxide will
alcohol, >99 % purity).
interfere and overestimate the amount of acidic material in the
9.3 Mixed Xylenes—(Warning—Flammable.) A technical
sample. In crude oils, bitumens, synthetic crude oils, and
grade of hydrocarbon that includes predominantly o-xylene,
subsequent fractions, the constituents that may be considered
m-xylene, p-xylene and lesser quantities of ethyl benzene. The
to have acidic characteristics include inorganic and organic
boiling point range is typically 136 °C to 140 °C.
acids, particularly naphthenic acids, phenolic compounds,
9.4 Titration Solvent—Add 250 mL 6 10 mL of anhydrous
resins, salts of heavy metals, and acid salts of polybasic acids.
isopropyl alcohol to 750 mL 6 10 mL of mixed xylenes.
7. Safety Precautions
Larger quantities of titration solvent can be prepared using
volumetric ratios (25:75) of the same proportions. Commer-
7.1 Wear chemical resistant gloves. Avoid excessive inha-
cially prepared reagent is also suitable.
lation of organic vapors and paraformaldehyde (flammable
solid) by working in a fume hood when possible. Pre-weighed 9.5 Toluene, pre-dissolution solvent (≥99 % purity).
paraformaldehyde packets should be substituted, if possible, to
9.6 Paraformaldehyde (polyoxymethylene)—(Warning—
minimize potential exposures when preparing titration slurry
Flammable solid. Harmful if inhaled. Irritant.) Terminated with
solutions. The titrant solution is corrosive and paraformalde-
hydroxyl end groups and in fine-powder form. Fine powder
hyde releases small quantities of formaldehyde during depo-
mesh is critical to successful and rapid analysis of the material.
lymerization.
Too large mesh reacts slowly, resulting in erroneous results. A
7.2 Consult Safety Data Sheets (SDS) for chemicals listed
sample of paraformaldehyde with greater than 80 % by mass
in this test method for further information.
having a mesh size between 100 and 200 was found suitable.
Avoid moist, aged, or oxidized reagent.
8. Apparatus
9.7 Benzoic acid (>99.9 % purity).
8.1 Automatic Titrator, capable of providing a dose rate of
9.8 n-heptane (≥99 % purity).
2 mL ⁄min.
9.9 Water—Type 1 deionized, or higher, as defined by
8.2 Thermistor, a device capable of measuring the tempera-
Specification D1193.
ture to 0.001 °C. The device should be immersed in the
titration solvent to a depth recommended by the manufacturer 9.10 Titration Slurry with Indicator—17 g 6 0.5 g of
and have a response time of less than 0.3 s. paraformaldehyde are added to one liter of titration solvent.
D8045 − 17 (2023)
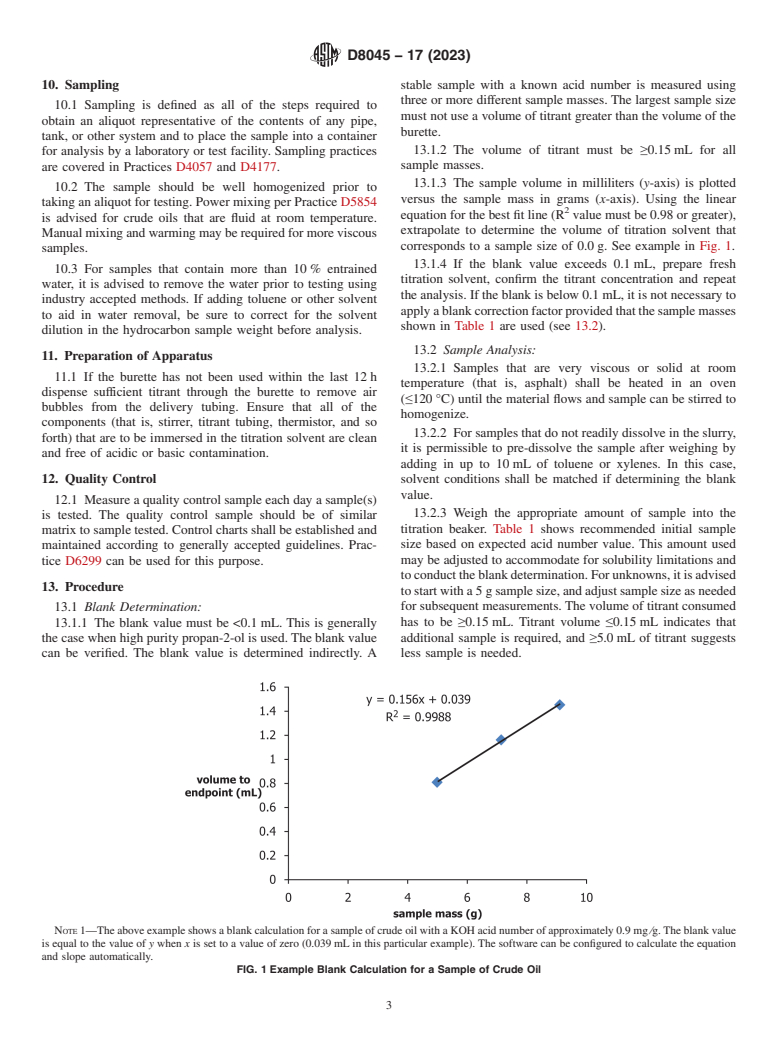
10. Sampling stable sample with a known acid number is measured using
three or more different sample masses. The largest sample size
10.1 Sampling is defined as all of the steps required to
must not use a volume of titrant greater than the volume of the
obtain an aliquot representative of the contents of any pipe,
burette.
tank, or other system and to place the sample into a container
13.1.2 The volume of titrant must be ≥0.15 mL for all
for analysis by a laboratory or test facility. Sampling practices
sample masses.
are covered in Practices D4057 and D4177.
13.1.3 The sample volume in milliliters (y-axis) is plotted
10.2 The sample should be well homogenized prior to
versus the sample mass in grams (x-axis). Using the linear
taking an aliquot for testing. Power mixing per Practice D5854
equation for the best fit line (R value must be 0.98 or greater),
is advised for crude oils that are fluid at room temperature.
extrapolate to determine the volume of titration solvent that
Manual mixing and warming may be required for more viscous
corresponds to a sample size of 0.0 g. See example in Fig. 1.
samples.
13.1.4 If the blank value exceeds 0.1 mL, prepare fresh
10.3 For samples that contain more than 10 % entrained
titration solvent, confirm the titrant concentration and repeat
water, it is advised to remove the water prior to testing using
the analysis. If the blank is below 0.1 mL, it is not necessary to
industry accepted methods. If adding toluene or other solvent
apply a blank correction factor provided that the sample masses
to aid in water removal, be sure to correct for the solvent
shown in Table 1 are used (see 13.2).
dilution in the hydrocarbon sample weight before analysis.
13.2 Sample Analysis:
11. Preparation of Apparatus
13.2.1 Samples that are very viscous or solid at room
11.1 If the burette has not been used within the last 12 h
temperature (that is, asphalt) shall be heated in an oven
dispense sufficient titrant through the burette to remove air
(≤120 °C) until the material flows and sample can be stirred to
bubbles from the delivery tubing. Ensure that all of the
homogenize.
components (that is, stirrer, titrant tubing, thermistor, and so
13.2.2 For samples that do not readily dissolve in the slurry,
forth) that are to be immersed in the titration solvent are clean
it is permissible to pre-dissolve the sample after weighing by
and free of acidic or basic contamination.
adding in up to 10 mL of toluene or xylenes. In this case,
12. Quality Control solvent conditions shall be matched if determining the blank
value.
12.1 Measure a quality control sample each day a sample(s)
13.2.3 Weigh the appropriate amount of sample into the
is tested. The quality control sample should be of similar
titration beaker. Table 1 shows recommended initial sample
matrix to sample tested. Control charts shall be established and
size based on expected acid number value. This amount used
maintained according to generally accepted guidelines. Prac-
may be adjusted to accommodate for solubility limitations and
tice D6299 can be used for this purpose.
to conduct the blank determination. For unknowns, it is advised
13. Procedure
to start with a 5 g sample size, and adjust sample size as needed
13.1 Blank Determination: for subsequent measurements. The volume of titrant consumed
13.1.1 The blank value must be <0.1 mL. This is generally has to be ≥0.15 mL. Titrant volume ≤0.15 mL indicates that
the case when high purity propan-2-ol is used. The blank value additional sample is required, and ≥5.0 mL of titrant suggests
can be verified. The blank value is determined indirectly. A less sample is needed.
NOTE 1—The above example shows a blank calculation for a sample of crude oil with a KOH acid number of approximately 0.9 mg ⁄g. The blank value
is equal to the value of y when x is set to a value of zero (0.039 mL in this particular example). The software can be configured to calculate the equation
and slope automatically.
FIG. 1 Example Blank Calculation for a Sample of Crude Oil
D8045 − 17 (2023)
TABLE 1 Recommended Sample Weights
automated delivery such that the same amount of paraformal-
Expected Acid Number Recommended Sample Mas
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.