ASTM E2898-20
(Guide)Standard Guide for Risk-Based Validation of Analytical Methods for PAT Applications
Standard Guide for Risk-Based Validation of Analytical Methods for PAT Applications
SIGNIFICANCE AND USE
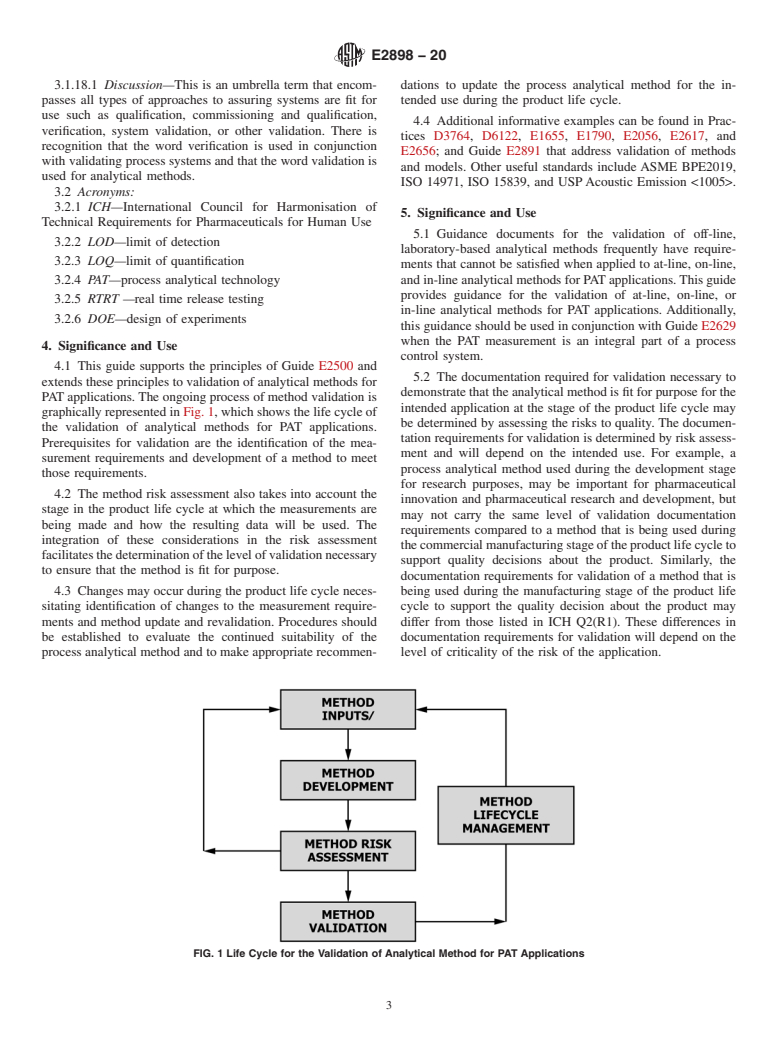
4.1 This guide supports the principles of Guide E2500 and extends these principles to validation of analytical methods for PAT applications. The ongoing process of method validation is graphically represented in Fig. 1, which shows the life cycle of the validation of analytical methods for PAT applications. Prerequisites for validation are the identification of the measurement requirements and development of a method to meet those requirements.
FIG. 1 Life Cycle for the Validation of Analytical Method for PAT Applications
4.2 The method risk assessment also takes into account the stage in the product life cycle at which the measurements are being made and how the resulting data will be used. The integration of these considerations in the risk assessment facilitates the determination of the level of validation necessary to ensure that the method is fit for purpose.
4.3 Changes may occur during the product life cycle necessitating identification of changes to the measurement requirements and method update and revalidation. Procedures should be established to evaluate the continued suitability of the process analytical method and to make appropriate recommendations to update the process analytical method for the intended use during the product life cycle.
4.4 Additional informative examples can be found in Practices D3764, D6122, E1655, E1790, E2056, E2617, and E2656; and Guide E2891 that address validation of methods and models. Other useful standards include ASME BPE2019, ISO 14971, ISO 15839, and USP Acoustic Emission .
SCOPE
1.1 This guide provides an overview to the risk-based validation of process analytical methods under a process analytical technology (PAT) paradigm for pharmaceuticals and biopharmaceuticals and as such includes guidance on assessing risk to product quality from inappropriate method validation.
1.2 This guide builds on existing standards on the topic of validation concentrating on applying such standards to analytical methods for on-line analysis. In particular, it addresses the validation of at-line, on-line, or in-line PAT measurements and covers both drug substance and drug product (DP) measurements.
1.3 The definitions of International Council for Harmonisation (ICH) validation parameters (such as specificity, precision, repeatability, etc.) apply; however, the method of demonstrating the validation parameters may vary from that described in ICH and is discussed.
1.4 As consistent with the U.S. Food and Drug Administration (FDA) process validation guidance, this document also briefly covers ongoing assurance that the method remains in a validated state during routine use.
1.5 Equipment and instrument qualification are out of the scope of this guide but will be referenced as inputs to validation of analytical methods for PAT applications.
1.6 The validation of multivariate prediction models is out of scope but will be referenced as inputs to validation of analytical methods for PAT applications.
1.6.1 The validation of any analytical model used in the PAT method is essential to the validation of the PAT method but, the details of the model validation process is out of scope. See term model validation, 3.1.7.
1.7 Microbiological methods are out of scope.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.9 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2898 − 20
Standard Guide for
Risk-Based Validation of Analytical Methods for PAT
1
Applications
This standard is issued under the fixed designation E2898; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.8 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
1.1 This guide provides an overview to the risk-based
responsibility of the user of this standard to establish appro-
validation of process analytical methods under a process
priate safety, health, and environmental practices and deter-
analytical technology (PAT) paradigm for pharmaceuticals and
mine the applicability of regulatory limitations prior to use.
biopharmaceuticalsandassuchincludesguidanceonassessing
1.9 This international standard was developed in accor-
risk to product quality from inappropriate method validation.
dance with internationally recognized principles on standard-
1.2 This guide builds on existing standards on the topic of
ization established in the Decision on Principles for the
validation concentrating on applying such standards to analyti-
Development of International Standards, Guides and Recom-
cal methods for on-line analysis. In particular, it addresses the
mendations issued by the World Trade Organization Technical
validation of at-line, on-line, or in-line PAT measurements and
Barriers to Trade (TBT) Committee.
covers both drug substance and drug product (DP) measure-
ments.
2. Referenced Documents
2
1.3 The definitions of International Council for Harmonisa-
2.1 ASTM Standards:
tion (ICH) validation parameters (such as specificity, precision,
D3764 Practice forValidation of the Performance of Process
repeatability, etc.) apply; however, the method of demonstrat-
Stream Analyzer Systems
ing the validation parameters may vary from that described in
D6122 Practice for Validation of the Performance of Multi-
ICH and is discussed.
variate Online, At-Line, and Laboratory Infrared Spectro-
photometer Based Analyzer Systems
1.4 As consistent with the U.S. Food and DrugAdministra-
E1655 Practices for Infrared Multivariate Quantitative
tion (FDA) process validation guidance, this document also
Analysis
briefly covers ongoing assurance that the method remains in a
E1790 Practice for Near Infrared Qualitative Analysis
validated state during routine use.
E2056 Practice for Qualifying Spectrometers and Spectro-
1.5 Equipment and instrument qualification are out of the
photometers for Use in Multivariate Analyses, Calibrated
scope of this guide but will be referenced as inputs to
Using Surrogate Mixtures
validation of analytical methods for PAT applications.
E2476 Guide for Risk Assessment and Risk Control as it
Impacts the Design, Development, and Operation of PAT
1.6 The validation of multivariate prediction models is out
of scope but will be referenced as inputs to validation of Processes for Pharmaceutical Manufacture
E2500 Guide for Specification, Design, and Verification of
analytical methods for PAT applications.
Pharmaceutical and Biopharmaceutical Manufacturing
1.6.1 ThevalidationofanyanalyticalmodelusedinthePAT
Systems and Equipment
method is essential to the validation of the PATmethod but, the
E2617 Practice for Validation of Empirically Derived Mul-
detailsofthemodelvalidationprocessisoutofscope.Seeterm
tivariate Calibrations
model validation, 3.1.7.
E2629 Guide for Verification of ProcessAnalytical Technol-
1.7 Microbiological methods are out of scope.
ogy (PAT) Enabled Control Systems
E2656 Practice for Real-time ReleaseTesting of Pharmaceu-
tical Water for the Total Organic Carbon Attribute
1
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture
ofPharmaceuticalandBiopharmaceuticalProductsandisthedirectresponsibilityof
Subcommittee E55.01 on Process Understanding and PAT System Management,
2
Implementation and Practice. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved May 15, 2020. Published June 2020. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 2013. Last previous edition approved in 2014 as E2898 – 14. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/E2898-20. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E2898 − 20
E2891 Guide for Multivariate Data Analysis in Pharmaceu- 3.1.7 model validation, n—the process of testing a ca
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E2898 − 14 E2898 − 20
Standard Guide for
Risk-Based Validation of Analytical Methods for PAT
1
Applications
This standard is issued under the fixed designation E2898; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide provides an overview to the risk-based validation of process analytical methods under a process analytical
technology (PAT) paradigm for pharmaceuticals and biopharmaceuticals and as such includes guidance on assessing risk to product
quality from inappropriate method validation.
1.2 This guide builds on existing standards on the topic of validation concentrating on applying such standards to analytical
methods for on-line analysis. In particular, it addresses the validation of at-line, on-line, or in-line PAT measurements and covers
both API and Drug Productdrug substance and drug product (DP) measurements.
1.3 The definitions of International Conference on HarmonizationCouncil for Harmonisation (ICH) validation parameters (such
as specificity, precision, repeatability, etc.) apply; however, the method of demonstrating the validation parameters may vary from
that described in ICH and is discussed.
1.4 As consistent with the U.S. Food and Drug Administration (FDA) process validation guidance, this document also briefly
covers ongoing assurance that the method remains in a validated state during routine use.
1.5 Equipment and instrument qualification are out of the scope of this guide but will be referenced as inputs to validation of
analytical methods for PAT applications.
1.6 The validation of multivariate prediction models is out of scope but will be referenced as inputs to validation of analytical
methods for PAT applications.
1.6.1 The validation of any analytical model used in the PAT method is essential to the validation of the PAT method but, the
details of the model validation process is out of scope. See term model validation,3.1.7.
1.7 Microbiological methods are out of scope.
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.9 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
D3764 Practice for Validation of the Performance of Process Stream Analyzer Systems
D6122 Practice for Validation of the Performance of Multivariate Online, At-Line, and Laboratory Infrared Spectrophotometer
Based Analyzer Systems
E1655 Practices for Infrared Multivariate Quantitative Analysis
E1790 Practice for Near Infrared Qualitative Analysis
1
This guide is under the jurisdiction of ASTM Committee E55 on Manufacture of Pharmaceutical and Biopharmaceutical Products and is the direct responsibility of
Subcommittee E55.01 on Process Understanding and PAT System Management, Implementation and Practice.
Current edition approved June 1, 2014May 15, 2020. Published June 2014June 2020. Originally approved in 2013. Last previous edition approved in 20132014 as E2898
– 13.14. DOI: 10.1520/E2898-14.10.1520/E2898-20.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E2898 − 20
E2056 Practice for Qualifying Spectrometers and Spectrophotometers for Use in Multivariate Analyses, Calibrated Using
Surrogate Mixtures
E2476 Guide for Risk Assessment and Risk Control as it Impacts the Design, Development, and Operation of PAT Processes
for Pharmaceutical Manufacture
E2500 Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing System
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.