ASTM D2022-89(2003)
(Test Method)Standard Test Methods of Sampling and Chemical Analysis of Chlorine-Containing Bleaches
Standard Test Methods of Sampling and Chemical Analysis of Chlorine-Containing Bleaches
SCOPE
1.1 These test methods cover the sampling and chemical analysis of chlorine-containing bleaches. The methods appear in the following order:
SectionsSodium Hypochlorite (Soda Bleach) Solutions:Sampling5Available Chlorine6-9Sodium Chlorate10-14Total Chlorine15-18Sodium Chloride19 and 20Total Alkalinity as Sodium Oxide (Na2O)21-24Free Alkali as Sodium Hydroxide (NaOH)25-28Calcium Hypochlorite:Sampling30Available Chlorine31-34Water35-40Chloroisocyanuric Acids and Their Derived Salts:Sampling42Available Chlorine (Iodometric-Thiosulfate Method)43-46Available Chlorine (Arsenite-Iodometric Method)47-50Moisture51-54
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Material Safety Data Sheets are available for reagents and materials. Review them for hazards prior to usage.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D2022–89(Reapproved 2003)
Standard Test Methods of
Sampling and Chemical Analysis of Chlorine-Containing
Bleaches
This standard is issued under the fixed designation D 2022; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope 2. Referenced Documents
1.1 These test methods cover the sampling and chemical 2.1 ASTM Standards:
analysis of chlorine-containing bleaches. The methods appear D 1193 Specification for Reagent Water
in the following order:
3. Terminology
Sections
Sodium Hypochlorite (Soda Bleach) Solutions:
3.1 Definitions:
Sampling 5
3.1.1 available chlorine—the measure of the oxidizing
Available Chlorine 6-9
powder of the chlorine present as hypochlorite. It is expressed
Sodium Chlorate 10-14
Total Chlorine 15-18
in terms of chlorine with a gram-equivalent weight of 35.46.
Sodium Chloride 19 and 20
Total Alkalinity as Sodium Oxide (Na O) 21-24
4. Reagents
Free Alkali as Sodium Hydroxide (NaOH) 25-28
Calcium Hypochlorite:
4.1 Purity of Reagents—Reagent grade chemicals shall be
Sampling 30
used in all tests. Unless otherwise indicated, it is intended that
Available Chlorine 31-34
all reagents shall conform to the specifications of the Commit-
Water 35-40
Chloroisocyanuric Acids and Their Derived Salts:
tee onAnalytical Reagents of theAmerican Chemical Society,
Sampling 42 3
where such specifications are available. Other grades may be
Available Chlorine (Iodometric—Thiosulfate Method) 43-46
used, provided it is first ascertained that the reagent is of
Available Chlorine (Arsenite—Iodometric Method) 47-50
Moisture 51-54
sufficiently high purity to permit its use without lessening the
accuracy of the determination.
1.2 This standard does not purport to address all of the
4.2 Unless otherwise indicated, references to water shall be
safety concerns, if any, associated with its use. It is the
understood to mean reagent water conforming to Specification
responsibility of the user of this standard to establish appro-
D 1193.
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. Material Safety
Data Sheets are available for reagents and materials. Review
them for hazards prior to usage.
Annual Book of ASTM Standards, Vol 11.01.
Reagent Chemicals, American Chemical Society Specifications, American
These test methods are under the jurisdiction of ASTM Committee D12 on Chemical Society, Washington, DC. For suggestions on the testing of reagents not
Soaps and Other Detergents and are the direct responsibility of Subcommittee listed by the American Chemical Society, see Analar Standards for Laboratory
D12.12 on Analysis of Soaps and Synthetic Detergents. Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
Current edition approved May 26, 1989. Published July 1989. Originally and National Formulary, U.S. Pharmacopeiall Convention, Inc. (USPC), Rockville,
published as D 2022 – 62 T. Last previous edition D 2022 – 87. MD.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2022–89 (2003)
SODIUM HYPOCHLORITE (SODABLEACH) SOLUTIONS
5. Sampling
where:
A =Na S O solution required for titration of the
5.1 The stability of soda bleach is influenced to a consider- 2 2 3
KIO solution, mL.
able degree by the purity of the alkali used in its preparation, 3
7.4 Starch Indicator Solution (0.5 %)—Mix 0.5 g of soluble
by the excess of alkali remaining, and by the kind and amount
starch with 5 mL of cold water and add to 95 mL of boiling
of metal contamination from equipment. Owing to the rela-
water. Mix, cool, and store in a sterilized bottle. Replace
tively unstable nature of bleach solutions, special attention
frequently or add 0.1 % salicylic acid to minimize deteriora-
shall be given to the collection and preservation of the sample.
tion.
Exposure to heat and sunlight promotes decomposition, and
shall be avoided. Samples shall be kept cool in a dark place (or
8. Procedure
in dark-colored bottles) until analyzed, which shall be done
8.1 Dissolve 2 to3gofKI crystals in 50 mL of water in a
without unnecessary delay.
250-mLErlenmeyer flask.Add 10 mLof acetic acid, then pipet
5.2 Strong solutions of bleach shall be accurately diluted
the aliquot of sample into the solution, keeping the tip of the
and aliquots taken for determination of available chlorine,
pipetbeneaththesurfaceofthesolutionuntildrained.Titrateat
chlorate, and total chlorine. The size of aliquots shall be such
once with 0.1 N Na S O solution until the iodine color is
that approximately 40 mLof the 0.1 N reagent is required. The 2 2 3
nearly gone, then add 1 mL of starch indicator solution and
alkali determinations shall be made directly on the sample
complete the titration to the disappearance of the blue color.
received and sample sizes to require about 10 mL of 0.1 N
Record the titration as A (see Section 14).
reagent are recommended.
5.3 Precision results will require sampling at a standard
9. Calculation
temperaturesuchas20°C.Resultsexpressedintermsofweight
9.1 Calculate the available chlorine as follows:
percent will require determination of the density or specific
gravity. This may be determined with a hydrometer or by
Available chlorine as Cl, g/L 5 ~AN 3 35.46!/V (2)
weighing the sample, after pipetting the amount to be diluted
Available chlorine as Cl, weight % 5 @~AN 3 0.03546!/VS# 3 100
for analysis into a tared weighing bottle. The weighed sample
may be transferred to a volumetric flask and used for subse-
9.2 Calculate the sodium hypochlorite content as follows:
quent analysis.
Sodium hypochlorite ~NaOCl!, g/L 5 ~AN 3 37.22!/V (3)
AVAILABLE CHLORINE
Sodium hypochlorite ~NaOCl!, weight %
5 @~AN 3 0.03722!/VS# 3 100
6. Summary of Test Method
6.1 The sample is added to an acidified solution of potas- where:
A =Na S O solution required for titration of the sample,
sium iodide and the released iodine is titrated with standard
2 2 3
mL
sodium thiosulfate solution to the usual starch end point.
N = normality of the Na S O solution,
2 2 3
V = original sample in aliquot used, mL, and
7. Reagents
S = specific gravity of the sample.
7.1 Acetic Acid, glacial.
7.2 Potassium Iodide (KI), crystals, iodate-free.
SODIUM CHLORATE
7.3 Sodium Thiosulfate Solution Standard, (0.1 N)—
Dissolve 25 g of sodium thiosulfate (Na S O·5H O) crystals
10. Summary of Test Method
2 2 3 2
in freshly boiled and cooled water and dilute to 1 L. The
10.1 Sodium chlorate is reduced with sodium bromide in 8
,
solution is more stable if the glassware is cleaned with 4 5
Nhydrochloricacid. Afterdilutionandadditionofpotassium
sulfuric-chromic acid and thoroughly rinsed with water. Stan-
iodide, the released iodine (equivalent to the hypochlorite plus
dardize against potassium iodate as follows: Weigh out accu-
chlorate) is titrated with standard sodium thiosulfate solution
rately 3.567 g of dry potassium iodate (KIO ) and transfer to a
and starch indicator.
1-L volumetric flask. Dissolve with water, make up to the
mark, and mix thoroughly.This solution will be exactly 0.1000
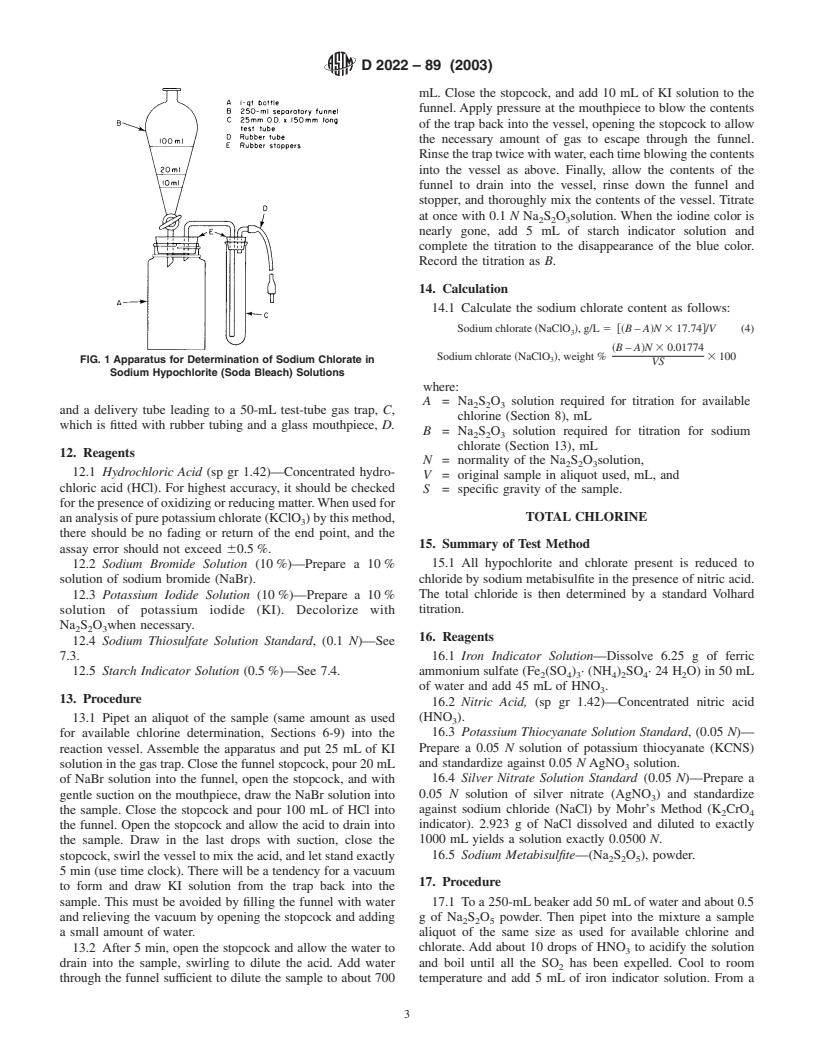
11. Apparatus
N. To standardize the Na S O solution, carefully pipet a 50-
2 2 3
11.1 The apparatus (Fig. 1) consists of 1-L wide-mouth
mL aliquot of the KIO solution into a 250-mL Erlenmeyer
reaction vessel, A (a 1-qt fruit jar will serve), fitted with a 2-
flask and dilute to 100 mL with water. Add1gofKI crystals.
hole rubber stopper carrying a separatory funnel, B, conve-
Whenitisdissolved,add15mLof1.0 Nhydrochloricacidand
niently graduated or marked at the 10, 20, and 100-mL levels,
titrate immediately with the Na S O solution. When the solu-
2 2 3
tion becomes light yellow, add 1 mL of starch indicator
solution and complete the titration to the disappearance of the
Ditz, Hugo, “Determination of Chlorates in Electrolytic Bleaching Lyes and in
Lyes Obtained from Absorption Vessels During the Production of Potassium
blue color. Standardize at least monthly. Calculate the normal-
Chlorate,” Chemiker Zeitung, Vol 25, 1901 p. 727.
ity of the Na S O solution as follows:
2 2 3 5
White, J. F., “Determination of Available Chlorine in Solutions Containing
Normality 5 ~50 3 0.1!/A (1) Textone (NaClO ),” American Dye-stuff Reporter, Vol 31, 1942 pp. 484–7.
D2022–89 (2003)
mL. Close the stopcock, and add 10 mL of KI solution to the
funnel. Apply pressure at the mouthpiece to blow the contents
of the trap back into the vessel, opening the stopcock to allow
the necessary amount of gas to escape through the funnel.
Rinsethetraptwicewithwater,eachtimeblowingthecontents
into the vessel as above. Finally, allow the contents of the
funnel to drain into the vessel, rinse down the funnel and
stopper, and thoroughly mix the contents of the vessel. Titrate
at once with 0.1 N Na S O solution. When the iodine color is
2 2 3
nearly gone, add 5 mL of starch indicator solution and
complete the titration to the disappearance of the blue color.
Record the titration as B.
14. Calculation
14.1 Calculate the sodium chlorate content as follows:
Sodium chlorate ~NaClO !, g/L 5 B – A N 3 17.74 /V (4)
@~ ! #
~B – A!N 3 0.01774
Sodium chlorate ~NaClO !, weight % 3 100
FIG. 1 Apparatus for Determination of Sodium Chlorate in
VS
Sodium Hypochlorite (Soda Bleach) Solutions
where:
A =Na S O solution required for titration for available
2 2 3
and a delivery tube leading to a 50-mL test-tube gas trap, C,
chlorine (Section 8), mL
which is fitted with rubber tubing and a glass mouthpiece, D.
B =Na S O solution required for titration for sodium
2 2 3
chlorate (Section 13), mL
12. Reagents
N = normality of the Na S O solution,
2 2 3
12.1 Hydrochloric Acid (sp gr 1.42)—Concentrated hydro-
V = original sample in aliquot used, mL, and
chloric acid (HCl). For highest accuracy, it should be checked
S = specific gravity of the sample.
forthepresenceofoxidizingorreducingmatter.Whenusedfor
TOTALCHLORINE
ananalysisofpurepotassiumchlorate(KClO )bythismethod,
there should be no fading or return of the end point, and the
15. Summary of Test Method
assay error should not exceed 60.5 %.
15.1 All hypochlorite and chlorate present is reduced to
12.2 Sodium Bromide Solution (10 %)—Prepare a 10 %
chloride by sodium metabisulfite in the presence of nitric acid.
solution of sodium bromide (NaBr).
12.3 Potassium Iodide Solution (10 %)—Prepare a 10 % The total chloride is then determined by a standard Volhard
titration.
solution of potassium iodide (KI). Decolorize with
Na S O when necessary.
2 2 3
16. Reagents
12.4 Sodium Thiosulfate Solution Standard, (0.1 N)—See
7.3. 16.1 Iron Indicator Solution—Dissolve 6.25 g of ferric
12.5 Starch Indicator Solution (0.5 %)—See 7.4. ammonium sulfate (Fe (SO ) · (NH ) SO ·24H O) in 50 mL
2 4 3 4 2 4 2
of water and add 45 mL of HNO .
13. Procedure
16.2 Nitric Acid, (sp gr 1.42)—Concentrated nitric acid
(HNO ).
13.1 Pipet an aliquot of the sample (same amount as used
16.3 Potassium Thiocyanate Solution Standard, (0.05 N)—
for available chlorine determination, Sections 6-9) into the
Prepare a 0.05 N solution of potassium thiocyanate (KCNS)
reaction vessel. Assemble the apparatus and put 25 mL of KI
solution in the gas trap. Close the funnel stopcock, pour 20 mL and standardize against 0.05 N AgNO solution.
16.4 Silver Nitrate Solution Standard (0.05 N)—Prepare a
of NaBr solution into the funnel, open the stopcock, and with
gentle suction on the mouthpiece, draw the NaBr solution into 0.05 N solution of silver nitrate (AgNO ) and standardize
against sodium chloride (NaCl) by Mohr’s Method (K CrO
the sample. Close the stopcock and pour 100 mL of HCl into
2 4
the funnel. Open the stopcock and allow the acid to drain into indicator). 2.923 g of NaCl dissolved and diluted to exactly
1000 mL yields a solution exactly 0.0500 N.
the sample. Draw in the last drops with suction, close the
stopcock, swirl the vessel to mix the acid, and let stand exactly 16.5 Sodium Metabisulfite—(Na S O ), powder.
2 2 5
5 min (use time clock). There will be a tendency for a vacuum
17. Procedure
to form and draw KI solution from the trap back into the
sample. This must be avoided by filling the funnel with water 17.1 To a 250-mLbeaker add 50 mLof water and about 0.5
and relieving the vacuum by opening the stopcock and adding gofNa S O powder. Then pipet into the mixture a sample
2 2 5
a small amount of water. aliquot of the same size as used for available chlorine and
13.2 After 5 min, open the stopcock and allow the water to chlorate. Add about 10 drops of HNO to acidify the solution
drain into the sample, swirling to dilute the acid. Add water and boil until all the SO has been expelled. Cool to room
through the funnel sufficient to dilute the sample to about 700 temperature and add 5 mL of iron indicator solution. From a
D2022–89 (2003)
buret add 0.5 mL of 0.05 N KCNS solution (Note 1). Then 22. Reagents
titrate to complete decolorization with 0.05 N AgNO solution.
22.1 HydrochloricAcid, Standard (0.1 N)—Prepare a 0.1 N
Filter off the precipitate by suction and wash three times with
solution of hydrochloric acid (HCl) and standardize against
water.Finally,back-titratethefiltrateandwashingswith0.05N
primary standard sodium carbonate and methyl red mixed
KCNS solution until a faint reddish color persists. For less
indicator.
accurate work the filtration may be avoided by adding 1 mLof
22.2 Hydrogen Peroxide Solution (3 %)—Prepare a 3 %
nitrobenzene to coagulate the suspension before back-titrating
solution of hydrogen peroxide (H O ).
2 2
the excess AgNO .
22.3 Methyl Red Mixed Indicator Solution—Dissolve 0.2 g
of methyl red in 100 mL of Formula 30 alcohol and 0.3 g
NOTE 1—This small amount of KCNS solution serves as an indicator to
bromcresol green in 300 mL of Formula 30 alcohol. Grinding
show when an excess of AgNO solution has been added. The back
of the methyl red may be necessary to ensure complete
titration is continued from the same buret and the total volume of KCNS
solution used is noted and used in the calculation.
solution. When reagents are completely dissolved, mix the two
solutions thoroughly.
18. Calculation
22.4 Sodium Hydroxide Solution (4 g/L)—Dissolve4gof
sodium hydroxide (NaOH) in water and dilute to 1 L.
18.1 Calculate the total chlorine content as follows:
Total Chlorine as Cl, g/L 5 @~CN ! – ~DN ! 3 35.46#/V (5) 23. Pr
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.