ASTM D2766-95(2009)
(Test Method)Standard Test Method for Specific Heat of Liquids and Solids (Withdrawn 2018)
Standard Test Method for Specific Heat of Liquids and Solids (Withdrawn 2018)
SIGNIFICANCE AND USE
The specific heat or heat capacity of a substance is a thermodynamic property that is a measure of the amount of energy required to produce a given temperature change within a unit quantity of that substance. It is used in engineering calculations that relate to the manner in which a given system may react to thermal stresses.
SCOPE
1.1 This test method covers the determination of the heat capacity of liquids and solids. It is applicable to liquids and solids that are chemically compatible with stainless steel, that have a vapor pressure less than 13.3 kPa (100 torr), and that do not undergo phase transformation throughout the range of test temperatures. The specific heat of materials with higher vapor pressures can be determined if their vapor pressures are known throughout the range of test temperatures.
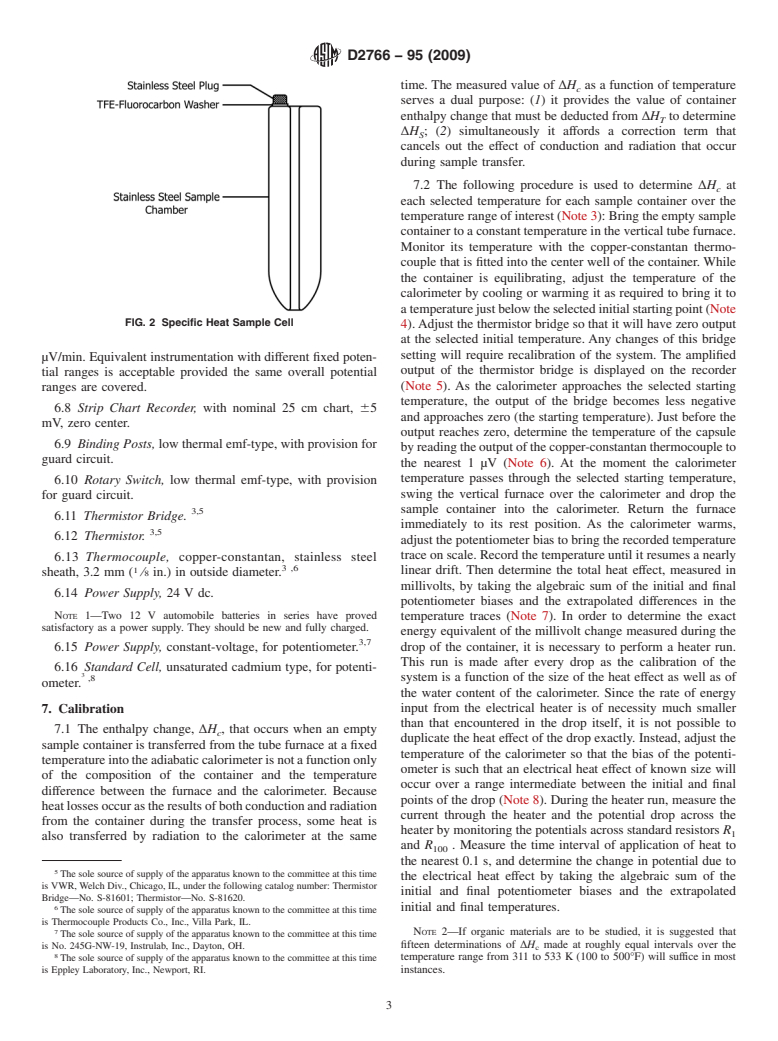
1.2 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
WITHDRAWN RATIONALE
This test method covers the determination of the heat capacity of liquids and solids. It is applicable to liquids and solids that are chemically compatible with stainless steel, that have a vapor pressure less than 13.3 kPa (100 torr), and that do not undergo phase transformation throughout the range of test temperatures. The specific heat of materials with higher vapor pressures can be determined if their vapor pressures are known throughout the range of test temperatures.
Formerly under the jurisdiction of Committee D02 on Petroleum Products, Liquid Fuels, and Lubricants, this test method was withdrawn in January 2018 in accordance with section 10.5.3.1 of the Regulations Governing ASTM Technical Committees, which requires that standards shall be updated by the end of the eighth year since the last approval date.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D2766 −95 (Reapproved 2009)
Standard Test Method for
1
Specific Heat of Liquids and Solids
This standard is issued under the fixed designation D2766; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope
T = temperature of hot zone, °C,
f
T = initial temperature of calorimeter, °C,
1.1 This test method covers the determination of the heat c
T' = T − T =temperature differential, °C,
f c
capacity of liquids and solids. It is applicable to liquids and
R = resistance of nominal 1-Ω standard resistor,
1
solids that are chemically compatible with stainless steel, that
R = resistance of nominal 100-Ω standard resistor,
100
haveavaporpressurelessthan13.3kPa(100torr),andthatdo
R = resistance of nominal 10 000-Ω standard resistor,
10 000
not undergo phase transformation throughout the range of test
E = emf across nominal 1-Ω standard resistor,
1
temperatures. The specific heat of materials with higher vapor
E = emf across nominal 100-Ω standard resistor,
100
pressurescanbedeterminediftheirvaporpressuresareknown
E = emf across nominal 10 000-Ω standard resistor,
10 000
throughout the range of test temperatures.
t = timeofapplicationofcalibrationheatercurrent,s,
c
q = total heat developed by calibration heater, cal,
1.2 The values stated in SI units are to be regarded as the
∆E = total heat effect for container, mV,
c
standard. The values given in parentheses are for information
∆E = total heat effect for sample+container, mV,
s
only.
∆e = total heat effect for calibration of calorimeter
c
1.3 This standard does not purport to address all of the
system during container run, mV,
safety concerns, if any, associated with its use. It is the
∆e = total heat effect for calibration of calorimeter
s
responsibility of the user of this standard to establish appro-
system during sample run, mV,
priate safety and health practices and determine the applica-
∆H = totalenthalpychangeforcontainerchangingfrom
c
bility of regulatory limitations prior to use.
T to T ,
f c
∆H = total enthalpy change for sample plus container
T
2. Referenced Documents
changing from T to T ,
f c
2
∆H = totalenthalpychangeforsamplechangingfromT
2.1 ASTM Standards:
s f
D1217Test Method for Density and Relative Density (Spe- to T ,
c
F = calorimeter factor,
cific Gravity) of Liquids by Bingham Pycnometer
W = weight of sample corrected for air buoyancy
3. Terminology d = density of sample at T,
f f
d = density of sample at T ,
c c
3.1 Definitions of Terms Specific to This Standard:
V = total volume of sample container,
T
3.1.1 specific heat—the ratio of the amount of heat needed
V = volume of sample vapor at T,
f f
to raise the temperature of a mass of the substance by a
V = volume of sample vapor at T ,
c c
specified amount to that required to raise the temperature of an
P = vapor pressure of sample at T,
f f
equal mass of water by the same amount, assuming no phase
P = vapor pressure of sample at T ,
c c
change in either case.
N = moles sample vapor at T,
f f
N = moles sample vapor at T ,
3.2 Symbols: c c
N = moles sample vapor condensed,
∆H = heat of vaporization of sample,
v
1
This test method is under jurisdiction ofASTM Committee D02 on Petroleum R = gas constant, and
Products, Liquid Fuels, and Lubricants and is the direct responsibility of Subcom-
K = heat of vaporization correction.
mittee D02.L0.07 on Engineering Sciences of High Performance Fluids and Solids
3.3 Units:
(Formally D02.1100).
Current edition approved Oct. 1, 2009. Published November 2009. Originally
3.3.1 The energy and thermal (heat) capacity units used in
approved in 1968. Last previous edition approved in 2005 as D2766–95(2005).
this method are defined as follows:
DOI: 10.1520/D2766-95R09.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
1 cal (International Table)=4.1868 J
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
1 Btu (British thermal unit, International Table)=
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. 1055.06 J
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
D2766−95 (2009)
1 Btu/lb °F=1 cal/g °C
1 Btu/lb °F=4.1868 J/g K
3.3.2 For all but the most precise measurements made with
this method the rounded-off value of 4.19 J/cal can be used as
this is adequate for the precision of the test and avoids the
difficulty caused by the dual definition of t
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.