ASTM E1578-93(1999)
(Guide)Standard Guide for Laboratory Information Management Systems (LIMS)
Standard Guide for Laboratory Information Management Systems (LIMS)
SCOPE
1.1 This guide describes computer systems used to manage laboratory information. The term Laboratory Information Management Systems (LIMS) describes this class of computer systems.
1.2 This guide covers LIMS ranging from small laboratories with simple requirements to large multi-site laboratories with complex requirements. The elements of the LIMS guide may be selected based on specific laboratory requirements.
1.3 The audience of this document includes: (1) end users of LIMS, (2) implementers of LIMS, (3) LIMS vendors, (4) instrument vendors, and (5) individuals who must approve LIMS funding.
1.4 The purpose of this guide includes: (1) help educate new users of Laboratory Information Management Systems (LIMS), (2) provide standard terminology that can be used by LIMS vendors and end users, (3) establish minimum requirements for primary LIMS functions, (4) provide guidance for the specification, evaluation, cost justification, implementation, project management, training, and documentation, and (5) provide an example of a LIMS function checklist.
1.5 Information contained in this guide will benefit a broad audience of people who work or interact with a laboratory. New LIMS users can use this guide to understand the purpose and functions of LIMS. The guide can help prospective LIMS users in understanding terminology, configurations, features, design, and costs. Individuals who are purchasing a LIMS can use this guide to identify functions that are recommended for specific laboratory environments. LIMS vendor Research and Development staffs can use the guide as a tool to evaluate, identify, and correct areas that need improvement. LIMS vendor sales staffs can use the guide to accurately represent functions of their LIMS product to prospective customers. This guide does not define laboratory instrument interfaces.
1.6 This guide can be used by laboratories of all sizes. The guide addresses complex issues that impact primarily large LIMS implementations. Small laboratories should review issues that may impact their environments. The implementation times and recommendations listed in this guide are directed at medium and large laboratories.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E1578–93(Reapproved1999)
Standard Guide for
Laboratory Information Management Systems (LIMS)
This standard is issued under the fixed designation E 1578; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope times and recommendations listed in this guide are directed at
medium and large laboratories.
1.1 This guide describes computer systems used to manage
laboratoryinformation.ThetermLaboratoryInformationMan-
2. Referenced Documents
agement Systems (LIMS) describes this class of computer
2.1 ASTM Standards:
systems.
E 622 Generic Guide for Computerized Systems
1.2 ThisguidecoversLIMSrangingfromsmalllaboratories
E 625 Guide for Training Users of Computerized Systems
with simple requirements to large multi-site laboratories with
E 627 Guide for Documenting Computerized Systems
complex requirements. The elements of the LIMS guide may
E 730 Guide for Developing Functional Designs for Com-
be selected based on specific laboratory requirements.
puterized Systems
1.3 Theaudienceofthisdocumentincludes:(1)endusersof
E 731 GuideforSelectionandAcquisitionofCommercially
LIMS, (2) implementers of LIMS, (3) LIMS vendors, (4)
Available Computerized Systems
instrument vendors, and (5) individuals who must approve
E 792 Guide for Computer Automation in the Clinical
LIMS funding.
Laboratory
1.4 The purpose of this guide includes: ( 1) help educate
E 919 Specification for Software Documentation for a
new users of Laboratory Information Management Systems
Computerized System
(LIMS), (2) provide standard terminology that can be used by
E 1013 Terminology Relating to Computerized Systems
LIMS vendors and end users, (3) establish minimum require-
E 1029 Guide for Documentation of Clinical Laboratory
ments for primary LIMS functions, (4) provide guidance for
Computer Systems
thespecification,evaluation,costjustification,implementation,
E 1340 Guide for Rapid Prototyping of Computerized Sys-
project management, training, and documentation, and (5)
tems
provide an example of a LIMS function checklist.
E 1381 Specification for Low-Level Protocol to Transfer
1.5 Information contained in this guide will benefit a broad
Messages Between Clinical Laboratory Instruments and
audience of people who work or interact with a laboratory.
Computer Systems
New LIMS users can use this guide to understand the purpose
E 1394 Specification for Transferring Information Between
and functions of LIMS. The guide can help prospective LIMS
Clinical Instruments and Computer Systems
users in understanding terminology, configurations, features,
2.2 IEEE Standards:
design, and costs. Individuals who are purchasing a LIMS can
100—Standard Dictionary of Electrical and Electronic
use this guide to identify functions that are recommended for
Terms
specific laboratory environments. LIMS vendor Research and
610—Standard Glossaries of Computer-Related Terminol-
Development staffs can use the guide as a tool to evaluate,
ogy
identify, and correct areas that need improvement. LIMS
729—Glossary of Software Engineering Terminology
vendor sales staffs can use the guide to accurately represent
730.1—Standard for Software Quality Assurance Plans
functions of their LIMS product to prospective customers.This
730.2—Guide for Software Quality Assurance Plans
guide does not define laboratory instrument interfaces.
828—Standard for Software Configuration Management
1.6 This guide can be used by laboratories of all sizes. The
Plans
guide addresses complex issues that impact primarily large
829—Standard for Software Test Documentation
LIMS implementations. Small laboratories should review is-
830—Guide to Software Requirements Specifications
sues that may impact their environments. The implementation
1008—Standard for Software Unit Testing
This guide is under the jurisdiction of ASTM Committee E13 on Molecular
Spectroscopy and Chromatography and is the direct responsibility of Subcommittee Annual Book of ASTM Standards, Vol 14.01.
E13.15 on Analytical Data. Available from IEEE, 445 Hoes Lane, P.O. Box 1331, Piscataway, NJ
Current edition approved Oct. 15, 1993. Published December 1993. 08855-1331.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E1578–93 (1999)
1012—Standard for Software Verification and Validation 3.3.4 data, n—record observations used for producing in-
Plans formation.
1016—Recommended Practice for Software Design De-
3.3.5 data analysis, n—the ability to display, manipulate,
scriptions transform, and verify LIMS database information.
1028—Standard for Software Reviews and Audits
3.3.6 data/information capture, v—the uni/bi-directional
1042—Guide to Software Configuration Management
communication of data/information to/from a LIMS.
1058.1—Standard for Software Project Management Plans
3.3.7 data integrity, n—the concept that information is not
1063—Standard for Software User Documentation
corrupted during communication, transfer, manipulation, stor-
1074—Standard for Developing Software Life Cycle Pro-
age, and recall functions.
cesses
3.3.8 determination, n—a single result, the lowest level of
1228—Standard for Software Safety Plans
information in a LIMS.
2.3 ANSI Standards:
3.3.8.1 Discussion—ALIMS example of a determination is
X3.172 American National Dictionary for Information Pro-
a pH result.
cessing Systems (ANDIS)
3.3.9 dynamictable(s),n—LIMSdatabasetable(s)orfile(s)
X3.135 Standard for Structured Query Language (SQL-2)
where sample and result information are stored.
X3.168 Standard for Embedding Structured Query Lan-
3.3.9.1 Discussion—The storage of LIMS sample and result
guage in Three GL Programs
data/information can be in one or more database tables.
2.4 ISO Standards:
Synonyms: LIMS database, active database.
InternationalStandardsOrganization(ISO)9000 Standards
3.3.10 event-triggering, v— action(s) performed following
2.5 Other Standards:
a specific condition(s).
Data Communication Standard for Chromatography
3.3.10.1 Discussion—Event triggering conditions can be
Data Communication Standard for Mass Spectrometry
initiated by way of data, process, or other external events.
CAALS-I Communication Specification
3.3.11 information, n—data plus context.
3.3.11.1 Discussion—Data are of little value without con-
3. Terminology
text.The information value of a LIMS is related not only to the
3.1 This guide defines terminology used in the LIMS field.
quality of data stored, but also the context or relationships that
Paragraph 3.3 defines LIMS terms specific to this guide.
are maintained within the system.
Paragraph 3.1 provides references to other computer-related
3.3.12 LIMS, n—acronym for Laboratory Information Man-
technical terms used in this guide. LIMS vendors use many
agement System. Computer application(s) [software] and hard-
different terms to define the items listed in 3.3. Users of this
ware that can acquire, analyze, report, and manage data and
document should request a terminology list from each vendor
information in the laboratory.
with a cross reference to the terms used in this guide.
3.3.13 laboratory management, n—the monitoring and
3.2 Definitions—For definitions of terms relating to com-
control of a laboratory’s data management, and to a lesser
puterized systems, refer to Terminology E 1013, Guide E 622,
degree, laboratory resources.
Glossaries IEEE 100, IEEE 610, IEEE 729, andANSI X3.172.
3.3.14 login, n—registration of a sample in a LIMS.
3.3 Definitions of Terms Specific to This Standard:
3.3.15 profile, n—a group of one or more tests.
3.3.1 archive (1), n—data from a working database that has
3.3.15.1 Discussion—A predefined list of tests that are
been transferred to storage media for long term storage.
assigned to a LIMS sample during login.
3.3.1.1 Discussion—Information stored in the archive can
3.3.16 raw data, n—the original record of an observation.
be retrieved for reporting or additional processing.
3.3.16.1 Discussion—Data entered into the system directly
3.3.2 archive (2), v—the process of making an archive (1).
from original observations (not from a source document) by
3.3.2.1 Discussion—Allows erasure of data from the work-
keyboard or automatically by laboratory test devices are
ing database in order to free space for additional data.
considered raw data. Raw data is recorded on laboratory
3.3.3 audit trail, n—a record of events related to a transac-
worksheets,memoranda,notes,notebooks,andaretheresultof
tion including the original information and any changes to the
original observations and activities related to laboratory test-
information.
ing. Raw data may include photographs, microfilms, computer
3.3.3.1 Discussion—The audit trail may be composed of
printouts, magnetic media, and recorded data from automated
manual or computerized records of events and information, or
instruments.
both. The audit trail is used to reconstruct a series of related
3.3.17 results, n—smallest unit of test data input into the
events that have occurred.
LIMS.
3.3.17.1 Discussion—For example, an individual pH result.
See determination.
Available from American Iron and Steel Institute (AISI), 1140 Connecticut
3.3.18 reporting, v—extracting, organizing, and presenting
Ave., Suite 705, Washington, DC 20036.
information stored in a LIMS.
Available from International Standards Organization, 1 Rue de Varembe, Case
Postale 56, Crt 1221, Geneva, Switzerland.
3.3.19 sample, n—a small part of portion of a material or
Available from Analytical Instrument Assoc., 225 Reinekers Lane, Suite 625,
product intended to be a representative of the whole.
Alexandria, VA 22314.
3.3.19.1 Discussion—A LIMS sample may be further sub-
Available from National Institute for Standards and Technology, Gaithersburg,
MD 20899. divided into sub samples or aliquots.
E1578–93 (1999)
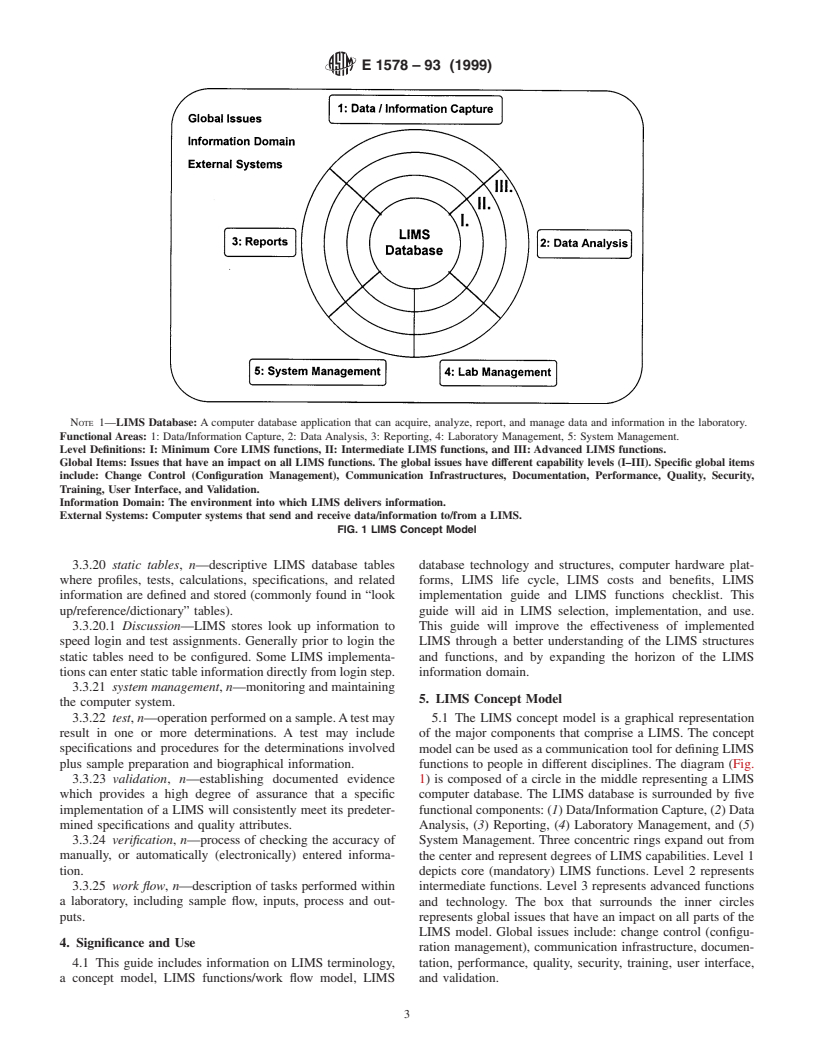
NOTE 1—LIMS Database: A computer database application that can acquire, analyze, report, and manage data and information in the laboratory.
Functional Areas: 1: Data/Information Capture, 2: Data Analysis, 3: Reporting, 4: Laboratory Management, 5: System Management.
Level Definitions: I: Minimum Core LIMS functions, II: Intermediate LIMS functions, and III: Advanced LIMS functions.
Global Items: Issues that have an impact on all LIMS functions. The global issues have different capability levels (I–III). Specific global items
include: Change Control (Configuration Management), Communication Infrastructures, Documentation, Performance, Quality, Security,
Training, User Interface, and Validation.
Information Domain: The environment into which LIMS delivers information.
External Systems: Computer systems that send and receive data/information to/from a LIMS.
FIG. 1 LIMS Concept Model
3.3.20 static tables, n—descriptive LIMS database tables database technology and structures, computer hardware plat-
where profiles, tests, calculations, specifications, and related forms, LIMS life cycle, LIMS costs and benefits, LIMS
information are defined and stored (commonly found in “look implementation guide and LIMS functions checklist. This
up/reference/dictionary” tables). guide will aid in LIMS selection, implementation, and use.
3.3.20.1 Discussion—LIMS stores look up information to This guide will improve the effectiveness of implemented
speed login and test assignments. Generally prior to login the LIMS through a better understanding of the LIMS structures
static tables need to be configured. Some LIMS implementa- and functions, and by expanding the horizon of the LIMS
tions can enter static table information directly from login step. information domain.
3.3.21 system management, n—monitoring and maintaining
5. LIMS Concept Model
the computer system.
3.3.22 test, n—operation performed on a sample.Atest may 5.1 The LIMS concept model is a graphical representation
result in one or more determinations. A test may include of the major components that comprise a LIMS. The concept
specifications and procedures for the determinations involved model can be used as a communication tool for defining LIMS
plus sample preparation and biographical information. functions to people in different disciplines. The diagram (Fig.
3.3.23 validation, n—establishing documented evidence 1) is composed of a circle in the middle representing a LIMS
which provides a high degree of assurance that a specific computer database. The LIMS database is surrounded by five
implementation of a LIMS will consistently meet its predeter- functional components: (1) Data/Information Capture, (2) Data
mined specifications and quality attributes. Analysis, (3) Reporting, (4) Laboratory Management, and (5)
3.3.24 verification, n—process of checking the accuracy of System Management. Three concentric rings expand out from
manually, or automatically (electronically) entered informa- the center and represent degrees of LIMS capabilities. Level 1
tion. depicts core (mandatory) LIMS functions. Level 2 represents
3.3.25 work flow, n—description of tasks performed within intermediate functions. Level 3 represents advanced functions
a laboratory, including sample flow, inputs, process and out-
and technology. The box that surrounds the inner circles
puts. represents global issues that have an impact on all parts of the
LIMS model. Global issues include: change control (configu-
4. Significance and Use
ration management), communication infrastructure, documen-
4.1 This guide includes information on LIMS terminology, tation, performance, quality, security, training, user interface,
a concept model, LIMS functions/work flow model, LIMS and validation.
E1578–93 (1999)
TABLE 1 LIMS Concept Model Sections
Level I—Minimum LIMS Functions
Global Issues LIMS Database Data/Information Capture Data Analysis Reporting Lab Management System Management
Change Control Manual Sample Login Result Verification Pre-Defined Reports Sample/Order Backup and Recovery
Status
Documentation Fixed Database Sample/Order
Structure Tracking
Quality Manual Result Entry Basic Calculations Sample Labels Backlog Report
Security Limited Capacity
User-Interface Limited Performance
Validation
Level II—Intermediate LIMS Functions
Global Issues LIMS Database Data/Information Capture Data Analysis Reporting Lab Management System Management
On-Line Documentation Intermediate Capacity On-Line from Comparison of Result User Defined Reports Scheduling of Lab Archiving
and Performance instruments (one-way
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.