ASTM D3154-00(2006)
(Test Method)Standard Test Method for Average Velocity in a Duct (Pitot Tube Method)
Standard Test Method for Average Velocity in a Duct (Pitot Tube Method)
SIGNIFICANCE AND USE
The procedures presented in this test method are available, in part, in Test Method D 3685/D 3685M, as well as the ASME Methods given in 2.3 and Footnote 8,5 the CFR given in 2.4, and the publication given in Footnote 9.6
SCOPE
1.1 This test method describes measurement of the average velocity of a gas stream for the purpose of determining gas flow in a stack, duct, or flue. Although technically complex, it is generally considered the most accurate and often the only practical test method for taking velocity measurements.
1.2 This test method is suitable for measuring gas velocities above 3 m/s (10 ft/s).
1.3 This test method provides procedures for determining stack gas composition and moisture content.
1.4 The values stated in SI units are to be regarded as standard. The inch-pound units given in parentheses are for information only.
1.5 This test method is applicable to conditions where steady-state flow occurs, and for constant fluid conditions. If these conditions are not meant, other methods must be used.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D3154 − 00(Reapproved 2006)
Standard Test Method for
Average Velocity in a Duct (Pitot Tube Method)
This standard is issued under the fixed designation D3154; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope D3685/D3685MTestMethodsforSamplingandDetermina-
tion of Particulate Matter in Stack Gases
1.1 This test method describes measurement of the average
D3796Practice for Calibration of Type S Pitot Tubes
velocity of a gas stream for the purpose of determining gas
E1Specification for ASTM Liquid-in-Glass Thermometers
flow in a stack, duct, or flue.Although technically complex, it
E337Test Method for Measuring Humidity with a Psy-
is generally considered the most accurate and often the only
chrometer (the Measurement of Wet- and Dry-Bulb Tem-
practical test method for taking velocity measurements.
peratures)
1.2 Thistestmethodissuitableformeasuringgasvelocities
2.2 EPA Standards:
above 3 m/s (10 ft/s).
EPA-600/9-76-005Quality Assurance Handbook for Air
1.3 This test method provides procedures for determining
Pollution Measurement Systems. Vol I. Principles
stack gas composition and moisture content.
EPA-600/4-77-027bQuality Assurance Handbook for Air
Pollution Measurement Systems. Vol. III. Stationary
1.4 The values stated in SI units are to be regarded as
Source Specific Methods
standard. The inch-pound units given in parentheses are for
information only. 2.3 ASME Standards:
ASME Performance Test Code: PTC 19.10-1968,Flue and
1.5 This test method is applicable to conditions where
Exhaust Gas Analysis
steady-state flow occurs, and for constant fluid conditions. If
ASME Performance Test Code: PTC 19.10-1981Part 10,
these conditions are not meant, other methods must be used.
Flue and Exhaust Measurements: Instruments and Appa-
1.6 This standard does not purport to address all of the 4
ratus
safety concerns, if any, associated with its use. It is the
ASME Performance Test Code: PTC 38-1980,Determining
responsibility of the user of this standard to establish appro- 4
the Concentration of Particulate Matter in a Gas Stream
priate safety and health practices and determine the applica-
2.4 Code of Federal Regulation:
bility of regulatory limitations prior to use.
CFR Part 50Standards of Performance for Stationary
Sources, Appendix A; Test Methods 1 through 4
2. Referenced Documents
2.1 ASTM Standards:
3. Terminology
D1071Test Methods for Volumetric Measurement of Gas-
3.1 Definitions:
eous Fuel Samples
3.1.1 For definitions of terms used in this test method, refer
D1193Specification for Reagent Water
to Terminology D1356.
D1356Terminology Relating to Sampling and Analysis of
3.2 Descriptions of Symbols Specific to This Standard:
Atmospheres
2 2
D3195Practice for Rotameter Calibration
A = cross-sectional area of stack, m (ft ).
D3631Test Methods for Measuring Surface Atmospheric
B = water vapor in the gas stream, proportion by
ws
Pressure
volume.
C = pitot tube coefficient, dimensionless.
p
D = internal diameter of stack, cm, (in.).
s
This test method is under the jurisdiction of ASTM Committee D22 on Air K = pitot tube constant:
p
1/2
Quality and is the direct responsibility of Subcommittee D22.03 on Ambient ~g/g2mol!
=
128.9m/s , (SI),
F G
Atmospheres and Source Emissions.
K
~ !
Current edition approved April 1, 2006. Published May 2006. Originally
approved in 1972. Last previous edition approved in 2000 as D3154-00. DOI:
10.1520/D3154-00R06.
2 3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or AvailablefromU.S.GovernmentPrintingOfficeSuperintendentofDocuments,
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401.
Standards volume information, refer to the standard’s Document Summary page on Available from American Society of Mechanical Engineers (ASME), ASME
the ASTM website. International Headquarters, Three Park Ave., New York, NY 10016-5990.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3154 − 00 (2006)
=
1/2
lb/lb 2mol
~ !
85.29ft/s
F G
~R!
, (inch-pound).
m = mean velocity, m/s (ft/s).
M = molecular weight of stack gas, dry basis,
d
g/g−mol (lb/lb−mol).
M = molecular weight of stack gas, wet basis,
s
g/g−mol (lb/lb−mol).
M = molecular weight of water, 18.0 g/g−mol FIG. 1 Pitot Tube
w
(18.0 lb/lb−mol).
N = number of sampling points across a diam-
W = initial mass of silica gel or silica gel plus
i
eter.
impinger, g.
n = nth sampling point from center of stack.
Y = dry gas meter calibration factor.
∆p = velocity head of stack gas, kPa (in. water).
0.28 = molecular weight of nitrogen or carbon
P = static pressure of stack gas, kPa (in. water).
static
monoxide, divided by 100.
P = barometric pressure, kPa (in. Hg).
bar
0.32 = molecularweightofoxygen,dividedby100.
P = absolute pressure at the dry gas meter (for
m
0.44 = molecular weight of carbon dioxide, divided
thistestmethoditequalsP ),kPa(in.Hg).
bar
by 100.
P = absolute stack gas pressure, kPa (mm Hg).
s
3600 = conversion factor, s/h.
P = standard ambient atmospheric pressure,
std
101.3 kPa (760 mm Hg).
4. Summary of Test Method
%CO = percentCO inthestackgas,byvolume,dry
2 2
4.1 This test method describes the use of instrumentation,
basis.
equipment, and operational procedures necessary for the mea-
%(N +CO) = sum of the percents of N and CO in the
2 2
surement and calculation of the average velocity of air or gas
stack gas, by volume, dry basis.
flowsinflues,ducts,orstacksutilizingthepitottubeprinciple,
%O = percent O in the stack gas, by volume, dry
2 2
with a manometer or draft gauge for pressure measurement.
basis.
The stack gas composition and moisture content are
Q = dry volumetric stack gas flow rate corrected
std
3 3
to standard conditions, dsm /h (dsft /h). determined, using an Orsat analyzer for composition, and
R = ideal gas constant, 0.08312 (kPa) (m )/g − condensation techniques for moisture.
mol) (K)−(SI system) or 21.85 (in. Hg)
5. Significance and Use
(ft )/(lb−mole) (°R)−(inch-pound).
r = radial distance from center of stack to nth
5.1 The procedures presented in this test method are
n
sampling point, cm (in.).
available, in part, in Test Method D3685/D3685M, as well as
ρ = density of water, 0.9971 g/mL (0.002194
w the ASME Methods given in 2.3 and Footnote 8, the CFR
lb/mL) at 25°C (77°F).
given in 2.4, and the publication given in Footnote 9.
S = between laboratory bias, m/s (ft/s).
T
S = among single laboratory bias, m/s (ft/s). 6. Apparatus
s
T = absolute average dry gas meter temperature,
m
6.1 Pitot Tube, used in conjunction with a suitable
K (°R).
manometer, provides the method for determining the velocity
T = stack gas temperature, K (° R).
s
in a duct. The construction of a standard pitot tube and the
T = standard absolute temperature, 298 K (537°
std
method of connecting it to a draft gauge are shown in Fig. 1.
R).
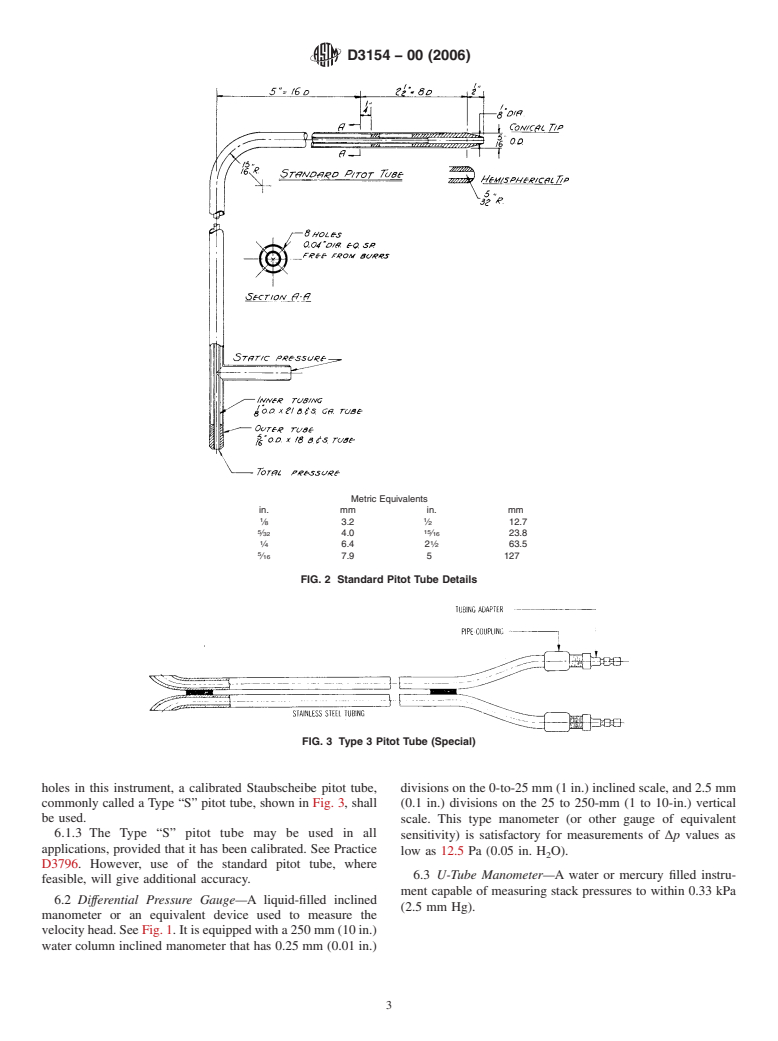
Details are shown in Fig. 2.
V = initial volume of condenser water, mL.
i
6.1.1 Tominimizethestemeffectwhenthephysicaldimen-
V = final volume of condenser water, mL.
f
sions of the pitot tube are too large with respect to the flow
V = volume of gas sample measured by the dry
m
3 3
scale, the diameter of the pitot tube barrel shall not exceed ⁄30
gas meter, dm (dft ).
the size of the duct diameter.
v = stack gas velocity, m/s (ft/s).
s
6.1.2 At locations where the standard pitot tube cannot be
V = volume of gas sample measured by the dry
m(std)
used in accordance with the sampling plan (see 8.1), or where
gas meter, corrected to standard conditions,
3 3
dust or moisture or both are present that may clog the small
dm (dft ).
V = volume of water vapor condensed, corrected
wc(std)
3 3
to standard conditions, sm (sft ).
Colen, P., Corey, R. C., and Meyers, J. W., “Methods and Instrumentation for
V = volumeofwatervaporcollectedinsilicagel,
wsg(std) Furnace Heat Absorption Studies; Temperature and Composition of Gases at
3 3
corrected to standard conditions, sm (sft ). FurnaceOutlets”TransactionoftheAmericanSocietyofMechanicalEngineers, 71,
pp. 965–78, 1949.
W = final mass of silica gel or silica gel plus
f
Bulletin WP-50, Western Precipitation Division, Joy Manufacturing Co.,
impinger, g.
“Methods for Determination of Velocity, Dust, and Mist Content of Gases.”
D3154 − 00 (2006)
Metric Equivalents
in. mm in. mm
1 1
⁄8 3.2 ⁄2 12.7
5 15
⁄32 4.0 ⁄16 23.8
1 1
⁄4 6.4 2 ⁄2 63.5
⁄16 7.9 5 127
FIG. 2 Standard Pitot Tube Details
FIG. 3 Type 3 Pitot Tube (Special)
holes in this instrument, a calibrated Staubscheibe pitot tube, divisions on the 0-to-25 mm (1 in.) inclined scale, and 2.5 mm
commonly called a Type “S” pitot tube, shown in Fig. 3, shall
(0.1 in.) divisions on the 25 to 250-mm (1 to 10-in.) vertical
be used.
scale. This type manometer (or other gauge of equivalent
6.1.3 The Type “S” pitot tube may be used in all
sensitivity) is satisfactory for measurements of ∆p values as
applications, provided that it has been calibrated. See Practice
low as 12.5 Pa (0.05 in. H O).
D3796. However, use of the standard pitot tube, where
6.3 U-Tube Manometer—A water or mercury filled instru-
feasible, will give additional accuracy.
ment capable of measuring stack pressures to within 0.33 kPa
6.2 Differential Pressure Gauge—A liquid-filled inclined
(2.5 mm Hg).
manometer or an equivalent device used to measure the
velocityhead.SeeFig.1.Itisequippedwitha250mm(10in.)
water column inclined manometer that has 0.25 mm (0.01 in.)
D3154 − 00 (2006)
FIG. 4 Integrated Gas Sampling Train
6.4 Thermocouple—A device for measuring temperature
utilizing the fact that a small voltage is generated whenever
two junctions of two dissimilar metals in an electric circuit are
FIG. 5 Orsat Apparatus
at different temperature levels.
6.4.1 Potentiometer—An instrument for measuring small
voltages, or for comparing small voltages with a known
6.6.7 Vacuum Gauge—Amercury manometer, or equivalent
voltage, used in conjuncture with the thermocouple.
of 101.3 kPa (760 mm Hg) capacity, to be used for the sample
6.4.2 Thermometer—An ASTM thermometer meeting the
train leak test. Test the gauge as described in 9.4.5.
requirements of Specification E1, for measuring the gas tem-
6.6.8 Orsat Gas Analyzer—See Fig. 5. The Orsat gas
peratures of small ducts.
analyzerisusedtoanalyzethegassampleforCO,O ,andCO
2 2
6.5 Mercury Barometer—An instrument capable of measur- stack gas concentrations, by successively passing the gas
ingambientatmosphericpressureto0.5kPa.SeeTestMethods through adsorbents that remove the specific gaseous compo-
D3631. nents. The difference in gas volumes before and after the
absorptions represents the amount of constituent gas in the
6.6 Gas Density Determination Equipment—See Fig. 4.
sample.
6.6.1 Probe—A stainless steel or borosilicate glass tube,
6.6.8.1 The analyzer shown in Fig. 5 includes a glass buret
equipped with an in-stack or out-of-stack filter to remove
to measure the gas volume of the sample, a water jacket to
particulate matter.
maintain constant temperature, a manifold to control the gas
6.6.2 Condenser—A water-cooled condenser that will not
flow, three absorption pipets (to remove CO,O , and CO),
2 2
removeO,CO ,CO,andN ,toremoveexcessmoistureifthe
2 2 2
rubber expansion bags, and a liquid-filled leveling bottle to
gas stream contains over 2% moisture by volume. The main
move the gas sample within the analyzer.
consideration is that the condenser volume be kept to the
6.6.8.2 For CO values >4%, a standard Orsat gas analyzer
minimum size because it will be more difficult to purge the
with a buret with 0.2 mL divisions and spacings divisions of
sample train before collecting a sample if the condenser is too
about 1 mm (0.14 in.) is satisfactory. For lower CO values or
large. A 63-mm (0.25-in.) stainless steel coil, or equivalent,
for O values >15%, a buret with 0.1 mL divisions with
connected to a water collection chamber with a capacity of
spacings of >1 mm shall be used.
about 40 mL is sufficient.
6.6.8.3 The analyzer shall be leak-tested before and after
6.6.3 Valve—A needle valve to adjust the sample gas flow
each test, as described in 9.4.1.1.
rate.
6.6.4 Pump—A leak-free diaphragm pump, to transport the 6.7 Gas Moisture Measuring Equipment— See Fig. 6.
sample gas to the flexible bag. A small surge tank shall be 6.7.1 Probe—See 6.6.1.
installed between the pump and the rate meter to eliminate the 6.7.1.1 ProbeHeater—Aheatingsystemtomaintaintheexit
pulsation effect of the pump on the rate meter. Leak-test the gas stack temperature at 120 6 14°C (250 6 25°F) during
pump, surge tank and rate meter (see 6.6.5), as described in sampling.
9.4.2. 6.7.1.2 The probe shall be checked for breaks and leaks
6.6.5 Rate Meter—A rotameter or equivalent rate meter, before each test, and the heater shall be checked to verify that
capableofmeasuringflowratestowithin 62%oftheselected it can maintain an exit air temperature of 100°C (212°F) when
flow rate. air is passed through the system at about 20 L/min (0.75
6.6.6 Flexible Bag—Aleak-freeinertplasticbag,havingthe ft /min).
capacity adequate for the selected flow rate and length of time 6.7.2 Condensers—Fourglassimpingersconnectedinseries
ofthetest.Acapacityof90L(3.2ft )isusuallysufficient.The with leak-free ground-glass fittings or equivalent leak-free
bag shall be leak-tested before each test, as described in 9.4.3. noncontaminating fittings.
D3154 − 00 (2006)
of the Committee on Analytical Reagents of the American
Chemical Society where such specifications are available.
7.2 Purity of Water—Water shall be Type 2 reagent water,
conforming to Specification D1193.
7.3 Alkaline Pyrogallic Acid Reagent, used as O absorp-
tion solution. Mix 40 mL of pyrogallic acid solution (see 7.9)
with 69 mLof KOH solution (see 7.8). Mix just before use. In
cold weather, some KOH may precipitate. If so, add enough
water to redissolve the KOH.
7.4 Confining Solution —Add 200 g of sodium sulfate
(Na SO ) (see 7.11) to 50 mL of concentrated sulfuric acid
2 4
(H SO ) (see 7.12), and add a few drops of methyl orange
2 4
indicator solution (see 7.7). Dilute to 1 L.
7.5 Cuprous Chloride Solution, (135 g/L)—Dissolve 180 g
FIG. 6 Moisture Sampling Train
of cuprous chloride (Cu Cl ) in 1 L of concentrated HCl (see
2 2
7.6).Add330mLofwater,andboilgentlyinalooselycovered
6.7.2.1 The first, th
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.