ASTM F1378-00
(Specification)Standard Specification for Shoulder Prosthesis
Standard Specification for Shoulder Prosthesis
SCOPE
1.1 This specification covers shoulder prostheses for total or hemiarthroplasty used to provide functioning articulation by employing glenoid and humeral components.
1.2 Devices for custom applications are not covered by this specification. Modular prostheses are included in this specification.
1.3 The values stated in SI are to be regarded as the standard. The inch-pound units given in parentheses are for information only.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 1378 – 00
Standard Specification for
Shoulder Prostheses
This standard is issued under the fixed designation F 1378; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope ods for Materials and Devices
F 799 Specification for Thermomechanically Processed
1.1 This specification covers shoulder prostheses for total or
Cobalt-Chromium-Molybdenum Alloy for Surgical Im-
hemiarthroplasty used to provide functioning articulation by
plants
employing glenoid and humeral components.
F 981 Practice for Assessment of Compatibility of Bio-
1.2 Devices for custom applications are not covered by this
Materials (Non-Porous) for Surgical Implants with Respect
specification. Modular prostheses are included in this specifi-
to Effect of Materials on Muscle and Bone
cation.
F 983 Practice for Permanent Marking of Orthopaedic Im-
1.3 The values stated in SI are to be regarded as the
plant Components
standard. The inch-pound units given in parentheses are for
F 1044 Test Method for Shear Testing of Porous Metal
information only.
Coatings
2. Referenced Documents F 1108 Specification for Ti6A14V Alloy Castings for Sur-
gical Implants
2.1 ASTM Standards:
F 1147 Test Method for Tension Testing of Porous Metal
F 75 Specification for Cast Cobalt-Chromium-Molybdenum
Coatings
Alloy for Surgical Implant Applications
F 1537 Specification for Wrought Cobalt-Chromium-
F 86 Practice for Surface Preparation and Marking of Me-
Molybdenum Alloy for Surgical Implants
tallic Surgical Implants
F 1829 Test Method for Static Evaluation of the Glenoid
F 90 Specification for Wrought Cobalt-Chromium-
Locking Mechanism in Shear
Tungsten-Nickel Alloy for Surgical Implant Application
F 2028 Test Methods for the Dynamic Evaluation of Gle-
F 136 Specification for Wrought Titanium 6A1-4V ELI
noid Loosening or Dissociation
Alloy for Surgical Implant Applications
2.2 ANSI Standard:
F 138 Specification for Stainless Steel Bar and Wire for
ASME B46.1–1995
Surgical Implants (Special Quality)
F 562 Specification for Wrought Cobalt-Nickel-Chromium-
3. Terminology
Molybdenum Alloy for Surgical Implant Applications
3.1 Definitions of Terms Specific to This Standard:
F 563 Specification for Wrought Cobalt-Nickel-Chromium-
3.1.1 collar—flange at junction of neck and stem.
Molybdenum-Tungsten-Iron Alloy for Surgical Implant
2 3.1.2 glenoid component—the prosthetic portion that re-
Applications
places, in part or in total, the glenoid fossa of the scapula and
F 603 Specification for High-Purity Dense Aluminum Ox-
2 articulates with the natural humeral head or a prosthetic
ide for Surgical Implant Applications
replacement.
F 648 Specification for Ultra-High-Molecular-Weight Poly-
3.1.3 head—bearing member for articulation with the gle-
ethylene Powder and Fabricated Form for Surgical Im-
noid.
plants
3.1.4 humeral component—the prosthetic portion that re-
F 745 Specification for Stainless Steel for Cast and
places, in part or in toto, the proximal humerus or humeral head
Solution-Annealed Surgical Implant Applications
and articulates with the natural glenoid fossa or a prosthetic
F 746 Test Method for Pitting or Crevice Corrosion of
replacement.
Metallic Surgical Implant Materials
3.1.5 keel, (or pegs)—single or multiple projections that
F 748 Practice for Selecting Generic Biological Test Meth-
provide resistance to translation or rotation of the glenoid
component, or both, by mating with cavities created in the
This specification is under the jurisdiction of ASTM Committee F04 on glenoid fossa.
Medical and Surgical Materials and Devices and is the direct responsibility of
Subcommittee F04.22 on Anthroplasty.
Current edition approved Nov. 10, 2000. Published January 2001. Originally
published as F 1378–92. Last previous edition F 1378–99. Available from American National Standards Institute, 11 W. 42nd St., 13th
Annual Book of ASTM Standards, Vol 13.01. Floor, New York, NY 10036.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F 1378
3.1.6 neck—segment connecting the head and the stem. Abduction shall be equal to or greater than 90°. Internal
3.1.7 stem—segment intended for insertion within the hu- rotation shall be equal to or greater than 90°. External rotation
meral medullary canal. shall be equal to or greater than 45°. Extension shall be equal
to or greater than 45°.
4. Classification
6.3 Porous metal coatings shall be tested according to Test
4.1 Constrained— A constrained joint prosthesis is used for
Method F 1044 (shear strength) and Test Method F 1147
joint replacement and resists dislocation of the prosthesis in
(tensile strength).
more than one anatomical plane and consists of either a single,
6.4 Guidelines for In-Vitro Laboratory Testing:
flexible, across-the-joint component or more than one compo-
6.4.1 Implant testing should reflect current clinical failures
nent linked together or affined.
and potential failure modes particular to the implant. These
4.2 Partially Constrained—A semi-constrained joint pros-
tests may be directed towards subluxation, glenoid loosening,
thesis is used for partial or total joint replacement and limits
insert disassociation from a metal backing, and humeral head
translation and rotation of the prosthesis in one or more planes
disassociation.
via the geometry of its articulating surfaces. It has no across-
6.4.2 All modular implants should be tested in accordance
the-joint linkages.
with Test Method F 1829.
4.3 Unconstrained— An unconstrained joint prosthesis is
6.4.3 All prosthetic glenoid components should be tested in
used for partial or total joint replacement and restricts mini-
accordance with Test Method F2028.
mally prosthesis movement in one or more planes. Its compo-
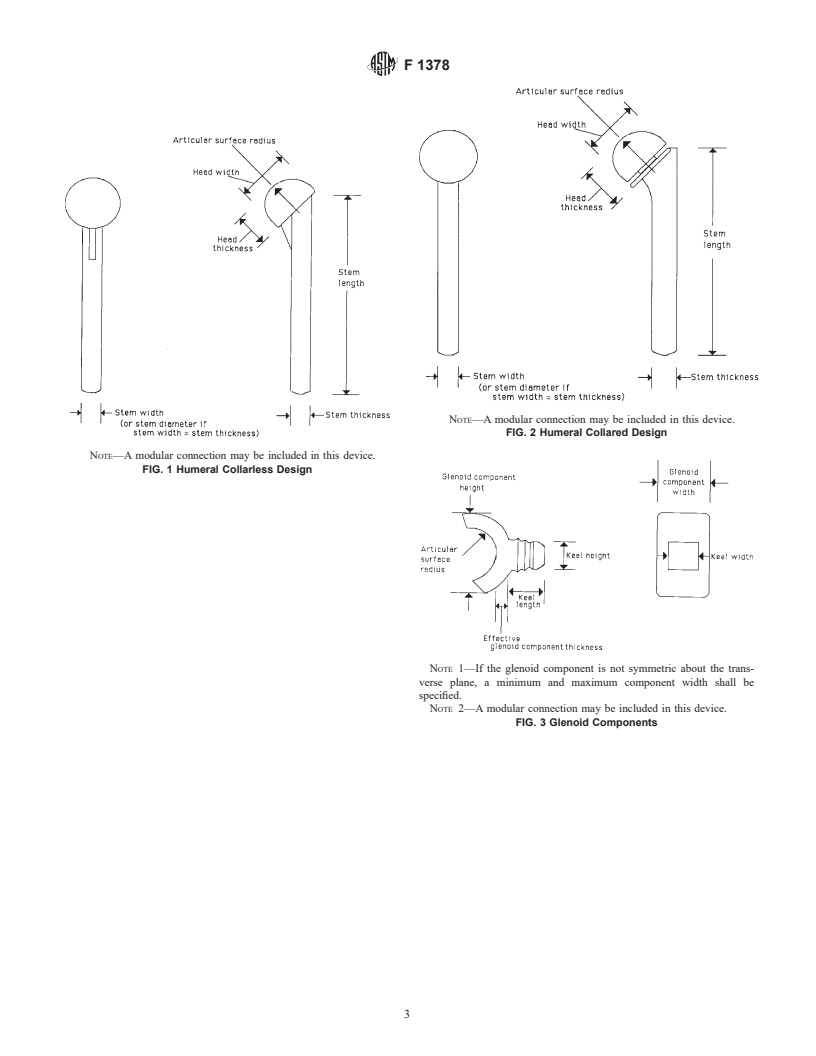
7. Dimensions
nents have no across-the-joint linkage.
7.1 Dimensions of shoulder joint replacement components
5. Materials and Manufacture
shall be as designated in Figs. 1-3.
5.1 The choice of materials is understood to be a necessary
but not sufficient ensurance of function of the device made
8. Finish and Product Marking
from them. All devices conforming to this specification shall be
8.1 Items conforming to this specification shall be finished
fabricated from materials, with adequate mechanical strength
and marked in accordance with Practice F 86, where appli-
and durability, corrosion resistance, and biocompatibility.
cable.
5.1.1 Mechanical Strength—Various components of shoul-
8.2 Articulating Surface Finishes:
der prostheses have been successfully fabricated from the
8.2.1 Metallic Bearing Surface— The main bearing surface
following materials. However, not all of these materials may
shall have a surface finish no rougher than 0.10 μm (4 μin.)
possess sufficient mechanical strength for critical highly-
roughness average, R , with a cutoff length of 0.25 mm, when
a
stressed components. See Specifications F 75, F 90, F 136,
measured according to the principles given in ASME
F 138, F 562, F 563 (nonbearing use only), F 603, F
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.