ASTM E2315-23
(Guide)Standard Guide for Assessment of Antimicrobial Activity Using a Time-Kill Procedure
Standard Guide for Assessment of Antimicrobial Activity Using a Time-Kill Procedure
SIGNIFICANCE AND USE
5.1 This procedure may be used to assess the in vitro reduction of a microbial population of test organisms after exposure to a test material.
SCOPE
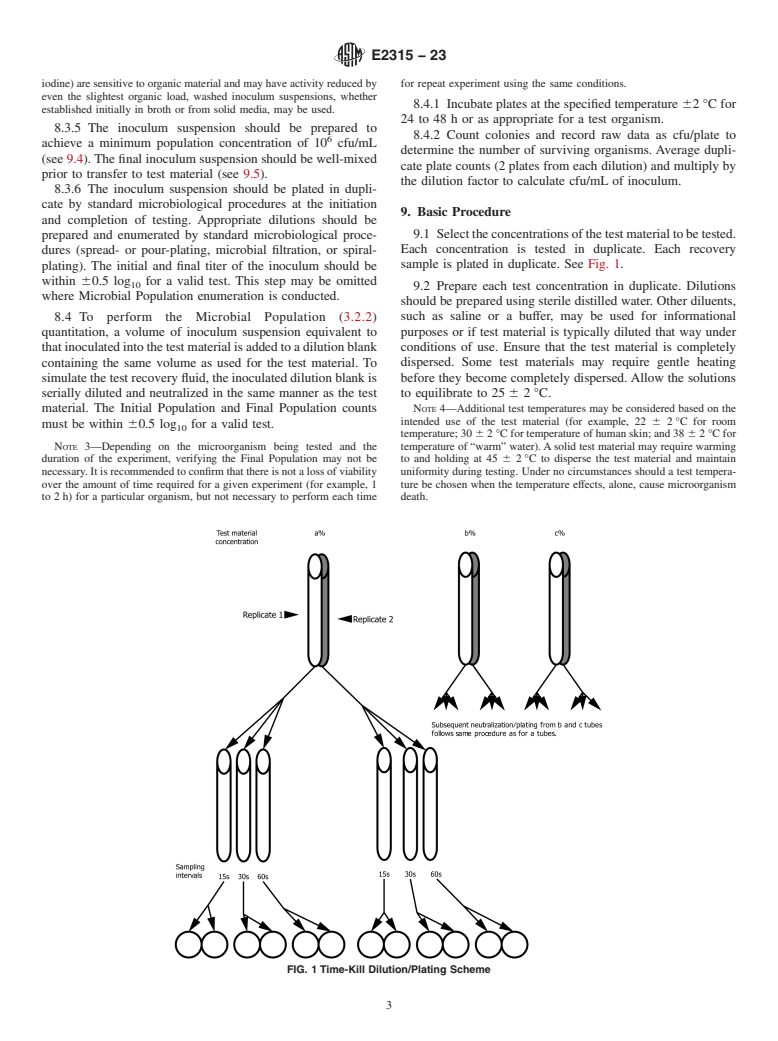
1.1 This guide covers an example of a method that measures the changes in a population of aerobic microorganisms within a specified sampling time when antimicrobial test materials are present.
1.1.1 Several options for organism selection and growth, inoculum preparation, sampling times and temperatures are provided.
1.1.2 When the technique is performed as a specific test method, it is critical that the above mentioned variables have been standardized.
1.1.3 Antimicrobial activity of specific materials, as measured by this technique, can vary significantly depending on variables selected.
1.1.4 Test Method E2783 may be referenced as an example of using fixed conditions and set variables to evaluate antimicrobial efficacy of water-miscible compounds.
1.1.5 This guide serves as a general teaching document for evaluating the antimicrobial activity using a variety of conditions to offer the flexibility needed in test conditions to cover a broad range of microorganisms and test substances.
1.1.6 It is important to understand the limitations of in vitro tests, especially comparisons of results from tests performed with different parameters. As an example, test results of microorganisms requiring growth supplements or special incubation conditions may not be directly comparable to organisms evaluated without those stated conditions.
1.2 Knowledge of microbiological techniques is required for this procedure.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E2315 − 23
Standard Guide for
Assessment of Antimicrobial Activity Using a Time-Kill
1
Procedure
This standard is issued under the fixed designation E2315; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope priate safety, health, and environmental practices and deter-
mine the applicability of regulatory limitations prior to use.
1.1 This guide covers an example of a method that measures
1.5 This international standard was developed in accor-
the changes in a population of aerobic microorganisms within
dance with internationally recognized principles on standard-
a specified sampling time when antimicrobial test materials are
ization established in the Decision on Principles for the
present.
Development of International Standards, Guides and Recom-
1.1.1 Several options for organism selection and growth,
mendations issued by the World Trade Organization Technical
inoculum preparation, sampling times and temperatures are
Barriers to Trade (TBT) Committee.
provided.
1.1.2 When the technique is performed as a specific test
2. Referenced Documents
method, it is critical that the above mentioned variables have
2
2.1 ASTM Standards:
been standardized.
D1193 Specification for Reagent Water
1.1.3 Antimicrobial activity of specific materials, as mea-
E1054 Practices for Evaluation of Inactivators of Antimicro-
sured by this technique, can vary significantly depending on
bial Agents
variables selected.
E2756 Terminology Relating to Antimicrobial and Antiviral
1.1.4 Test Method E2783 may be referenced as an example
Agents
of using fixed conditions and set variables to evaluate antimi-
E2783 Test Method for Assessment of Antimicrobial Activ-
crobial efficacy of water-miscible compounds.
ity for Water Miscible Compounds Using a Time-Kill
1.1.5 This guide serves as a general teaching document for
Procedure
evaluating the antimicrobial activity using a variety of condi-
tions to offer the flexibility needed in test conditions to cover
3. Terminology
a broad range of microorganisms and test substances.
3.1 Definitions:
1.1.6 It is important to understand the limitations of in vitro
3.1.1 For definitions of standard terms relating to antimi-
tests, especially comparisons of results from tests performed
crobial agents used in this guide, refer to Terminology E2756.
with different parameters. As an example, test results of
3.2 Definitions of Terms Specific to This Standard:
microorganisms requiring growth supplements or special incu-
3.2.1 inoculum suspension, n—the initial suspension of test
bation conditions may not be directly comparable to organisms
organism used to inoculate the test material. This may also be
evaluated without those stated conditions.
known as the organism inoculum (see 8.3).
1.2 Knowledge of microbiological techniques is required
3.2.2 microbial population, n—the microbial count (cfu/
for this procedure.
mL) in the final volume of test material (see 9.4). This may also
1.3 The values stated in SI units are to be regarded as
be known as the “numbers control.” The measurement may be
standard. No other units of measurement are included in this
taken at time zero which may be termed “Initial Population.”
standard.
Alternatively, the measurement may be taken at each exposure
time or the longest exposure time used during testing to
1.4 This standard does not purport to address all of the
simulate the test procedure which may be termed “Final
safety concerns, if any, associated with its use. It is the
Population.”
responsibility of the user of this standard to establish appro-
3.2.3 neutralization, n—the process for inactivating or
quenching the activity of a test material. This may be achieved
1
This guide is under the jurisdiction of ASTM Committee E35 on Pesticides,
Antimicrobials, and Alternative Control Agents and is the direct responsibility of
2
Subcommittee E35.15 on Antimicrobial Agents. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved April 1, 2023. Published May 2023. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 2003. Last previous edition approved in 2016 as E2315 – 16. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/E2315-23. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
----------------------
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E2315 − 16 E2315 − 23
Standard Guide for
Assessment of Antimicrobial Activity Using a Time-Kill
1
Procedure
This standard is issued under the fixed designation E2315; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide covers an example of a method that measures the changes in a population of aerobic microorganisms within a
specified sampling time when antimicrobial test materials are present. Several options for organism selection and growth, inoculum
preparation, sampling times and temperatures are provided. When the technique is performed as a specific test method, it is critical
that the above mentioned variables have been standardized. Antimicrobial activity of specific materials, as measured by this
technique, may vary significantly depending on variables selected. It is important to understand the limitations of in vitro tests,
especially comparisons of results from tests performed with different parameters. As an example, test results of microorganisms
requiring growth supplements or special incubation conditions may not be directly comparable to organisms evaluated without
those stated conditions.
1.1.1 Several options for organism selection and growth, inoculum preparation, sampling times and temperatures are provided.
1.1.2 When the technique is performed as a specific test method, it is critical that the above mentioned variables have been
standardized.
1.1.3 Antimicrobial activity of specific materials, as measured by this technique, can vary significantly depending on variables
selected.
1.1.4 Test Method E2783 may be referenced as an example of using fixed conditions and set variables to evaluate antimicrobial
efficacy of water-miscible compounds.
1.1.5 This guide serves as a general teaching document for evaluating the antimicrobial activity using a variety of conditions to
offer the flexibility needed in test conditions to cover a broad range of microorganisms and test substances.
1.1.6 It is important to understand the limitations of in vitro tests, especially comparisons of results from tests performed with
different parameters. As an example, test results of microorganisms requiring growth supplements or special incubation conditions
may not be directly comparable to organisms evaluated without those stated conditions.
1.2 Knowledge of microbiological techniques is required for this procedure.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1
This guide is under the jurisdiction of ASTM Committee E35 on Pesticides, Antimicrobials, and Alternative Control Agents and is the direct responsibility of
Subcommittee E35.15 on Antimicrobial Agents.
Current edition approved Jan. 15, 2016April 1, 2023. Published March 2016May 2023. Originally approved in 2003. Last previous edition approved in 20082016 as
E2315 – 03E2315 – 16.(2008). DOI: 10.1520/E2315-16.10.1520/E2315-23.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
E2315 − 23
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and healthsafety, health, and environmental practices and determine
the applicability of regulatory requirementslimitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
D1193 Specification for Reagent Water
E1054 Practices for Evaluation of Inactivators of Antimicrobial Agents
E2756 Terminology Relating to Antimicrobial and Antiviral Agents
E2783 Test Method for Assessment of Antimicrobial Activity for Water Miscible Compounds Using a Time-Kill Procedure
3. Terminology
3.1 Definitions:
3.1.1 For definitions of standard terms relating to antimicrobial agents used in this guide, refer to Terminology E2756.
3.2 Definitions:Definitions of Terms Speci
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.