ASTM D7818-23
(Test Method)Standard Test Method for Enumeration of Proteolytic Bacteria in Fresh (Uncured) Hides and Skins
Standard Test Method for Enumeration of Proteolytic Bacteria in Fresh (Uncured) Hides and Skins
SIGNIFICANCE AND USE
4.1 This test method enumerates proteolytic bacteria. Proteolytic bacteria have been known to cause damage to hides and skins.
SCOPE
1.1 This test method covers the enumeration of bacteria that can hydrolyze protein/collagen in fresh (uncured) hides and skins. This test method is applicable to uncured hides and skins.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D7818 − 23
Standard Test Method for
Enumeration of Proteolytic Bacteria in Fresh (Uncured)
1
Hides and Skins
This standard is issued under the fixed designation D7818; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
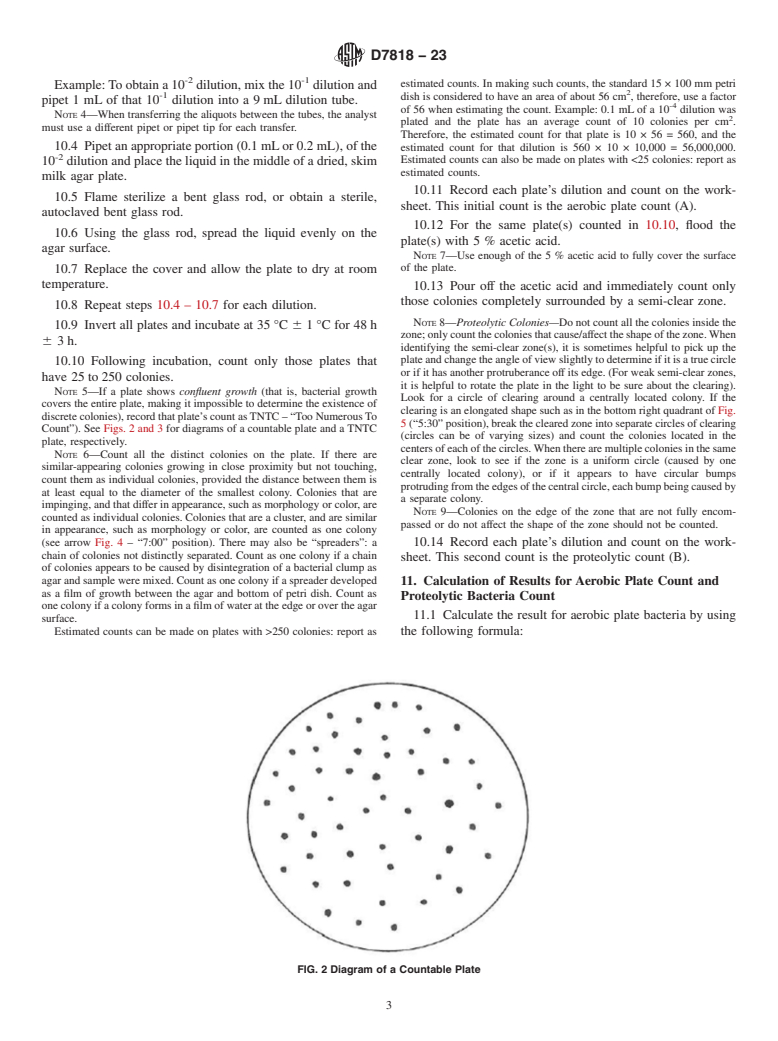
1. Scope plates are incubated under aerobic conditions at 35 °C for 48 h.
After incubation, to determine bacteria that can hydrolyze
1.1 This test method covers the enumeration of bacteria that
protein (proteolytic), the plates are flooded with dilute acid and
can hydrolyze protein/collagen in fresh (uncured) hides and
the colonies showing a halo are counted.
skins. This test method is applicable to uncured hides and
skins.
4. Significance and Use
1.2 The values stated in SI units are to be regarded as
4.1 This test method enumerates proteolytic bacteria. Pro-
standard. No other units of measurement are included in this
teolytic bacteria have been known to cause damage to hides
standard.
and skins.
1.3 This standard does not purport to address all of the
5. Apparatus
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
5.1 Incubator, 35 °C 6 1 °C.
priate safety, health, and environmental practices and deter-
5.2 Colony counter—(not mandatory, but highly recom-
mine the applicability of regulatory limitations prior to use.
mended).
1.4 This international standard was developed in accor-
5.3 Sterile pipets.
dance with internationally recognized principles on standard-
ization established in the Decision on Principles for the
5.4 Bent glass rods, sterile.
Development of International Standards, Guides and Recom-
5.5 Stomacher, for mixing initial dilution. (If stomacher is
mendations issued by the World Trade Organization Technical
unavailable, hand-mix.)
Barriers to Trade (TBT) Committee.
5.6 Balance.
2. Referenced Documents
5.7 Sterile petri dishes.
2
2.1 ASTM Standards:
5.8 Autoclave (sterilizer). (Check the effectiveness of ster-
D6715 Practice for Sampling and Preparation of Fresh or
ilization weekly. For example, place spore suspensions or strips
Salt-Preserved (Cured) Hides and Skins for Chemical and
of Bacillus stearothermophilus (commercially available) inside
Physical Tests
glassware for a full autoclave cycle. Follow manufacturer’s
E691 Practice for Conducting an Interlaboratory Study to
directions for sterilization of specific media.)
Determine the Precision of a Test Method
5.9 Stomacher bags, or sterile, sealable quart plastic bag
E177 Practice for Use of the Terms Precision and Bias in
(for example, food storage type, sterile bag).
ASTM Test Methods
5.10 Cutting tool, sterile (for example, scalpel blade and
3. Summary of Test Method
forcep, as needed for cutting fresh hides and skins).
3.1 Samples of uncured hides and skins are serially diluted
5.11 Vortex mixer, for mixing dilution tubes (optional).
and plated on agar containing casein from skim milk. The
5.12 pH meter.
5.13 Waterbath, 45 °C 6 1 °C.
1
This test method is under the jurisdiction of ASTM Committee D31 on Leather
and is the direct responsibility of Subcommittee D31.02 on Wet Blue.
5.14 Autoclave thermometer.
Current edition approved Sept. 1, 2023. Published September 2023. Originally
approved in 2012. Last previous edition approved in 2021 as D7818 – 12 (2021).
6. Reagents and Materials
DOI: 10.1520/D7818-23.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or 6.1 5 % acetic acid.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
6.2 Butterfield’s Phosphate Stock Solution: Dissolve 34 g
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. KH PO (Potassium Phosphate monobasic) in 500 mL DI
2 4
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
D7818 − 23
water. Adjust the pH to 7.2 6 0.1 with 1N – 6N NaOH. Bring 9.2 Autoclave the prepared agar for 15 min at 121 °C. (See
volume to 1 L with DI water. Sterilize for 15 min at 121 °C. Note 1.)
NOTE 1—Typical autoclave setting is 120 °C to 124 °C. (See 5.8.)
9.3 Prepare a 10 % powdered skim milk mixture by adding
6.3 Butterfield’s Phosphate Diluent (BPD): Take 1.25 mL of
10 g powdered skim milk to 100 mL DI water, then stirring the
Butterfield’s Phosphate Stock solution (6.2) and bring to 1 L
mixture to dissolve it. Autoclave
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D7818 − 12 (Reapproved 2021) D7818 − 23
Standard Test Method for
Enumeration of Proteolytic Bacteria in Fresh (Uncured)
1
Hides and Skins
This standard is issued under the fixed designation D7818; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers the enumeration of bacteria that can hydrolyze protein/collagen in fresh (uncured) hides and skins.
This test method is applicable to uncured hides and skins.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
D6715 Practice for Sampling and Preparation of Fresh or Salt-Preserved (Cured) Hides and Skins for Chemical and Physical
Tests
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
3. Summary of Test Method
3.1 Samples of uncured hides and skins are serially diluted and plated on agar containing casein from skim milk. The plates are
incubated under aerobic conditions at 35 °C 35 °C for 48 h. After incubation, to determine bacteria that can hydrolyze protein
(proteolytic), the plates are flooded with dilute acid and the colonies showing a halo are counted.
4. Significance and Use
4.1 This test method enumerates proteolytic bacteria. Proteolytic bacteria have been known to cause damage to hides and skins.
5. Apparatus
5.1 Incubator, 35 6 1 °C.35 °C 6 1 °C.
1
This test method is under the jurisdiction of ASTM Committee D31 on Leather and is the direct responsibility of Subcommittee D31.02 on Wet Blue.
Current edition approved Sept. 1, 2021Sept. 1, 2023. Published October 2021September 2023. Originally approved in 2012. Last previous edition approved in 20162021
as D7818 – 12 (2016).(2021). DOI: 10.1520/D7818-12R21.10.1520/D7818-23.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
D7818 − 23
5.2 Colony counter—(not mandatory, but highly recommended).
5.3 Sterile pipets.
5.4 Bent glass rods, sterile.
5.5 Stomacher, for mixing initial dilution. (If stomacher is unavailable, hand-mix.)
5.6 Balance.
5.7 Sterile petri dishes.
5.8 Autoclave (sterilizer). (Check the effectiveness of sterilization weekly. For example, place spore suspensions or strips of
Bacillus stearothermophilus (commercially available) inside glassware for a full autoclave cycle. Follow manufacturer’s directions
for sterilization of specific media.)
5.9 Stomacher bags, or sterile, sealable quart plastic bag (for example, food storage type, sterile bag).
5.10 Cutting tool, sterile (for example, scalpel blade and forcep, as needed for cutting fresh hides and skins).
5.11 Vortex mixer, for mixing dilution tubes (optional).
5.12 pH meter.
5.13 Waterbath, 45 6 1 °C.45 °C 6 1 °C.
5.14 Autoclave thermometer.
6. Reagents and Materials
6.1 5 % acetic acid.
6.2 Butterfield’s Phosphate Stock Solution: Dissolve 34 g KH PO (Potassium Phosphate monobasic) in 500 mL 500 mL DI water.
2 4
Adjust the pH to 7.2 6 0.1 with 1N – 6N NaOH. Bring volume to 1 L with DI water. Sterilize for 15 min at 121 °C.121 °C.
NOTE 1—Typical autoclave setting is 120 – 124 °C. 120 °C to 124 °C. (See 5.8.)
6.3 Butterfield’s Phosphate Diluent (BPD): Take 1.25 mL 1.25 mL of Butterfield’s Phosphate Stock
...










Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.