ASTM F1781-97
(Specification)Standard Specification for Elastomeric Flexible Hinge Finger Total Joint Implants
Standard Specification for Elastomeric Flexible Hinge Finger Total Joint Implants
SCOPE
1.1 This specification covers elastomeric flexible hinge finger total joint implants, used with and without metal grommets in the reconstruction of the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints.
1.2 This specification excludes those implants that do not have an across-the-joint elastomeric linkage. The specification is limited to implants made from one material in a single one-step molding procedure.
1.3 The values stated in SI units are to be regarded as standard. The inch-pound units given in parentheses are for information only.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 1781 – 97
Standard Specification for
Elastomeric Flexible Hinge Finger Total Joint Implants
This standard is issued under the fixed designation F 1781; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope Materials on Muscle and Bone
F 983 Practice for Permanent Marking of Orthopaedic Im-
1.1 This specification covers elastomeric flexible hinge
plant Components
finger total joint implants, used with and without metal
2.2 Government Standards:
grommets in the reconstruction of the metacarpophalangeal
21 CFR Part 820 Good Manufacturing Practices for Medi-
(MCP) and proximal interphalangeal (PIP) joints.
cal Devices
1.2 This specification excludes those implants that do not
MIL STD 177A Rubber Products, Terms for Visible De-
have an across-the-joint elastomeric linkage. The specification
fects
is limited to implants made from one material in a single
2.3 Other Standard:
one-step molding procedure.
EN 30993-1 Biological Evaluations of Medical Devices
1.3 The values stated in SI units are to be regarded as
Part 1: Guidance on Selection of Tests
standard. The inch-pound units given in parentheses are for
information only.
3. Significance and Use
2. Referenced Documents 3.1 The prostheses described in this specification are in-
tended for use in the proximal interphalangeal (PIP) and
2.1 ASTM Standards:
metacarpophalangeal (MCP) joints.
D 412 Test Methods for Vulcanized Rubber and Thermo-
plastic Rubbers and Thermoplastic Elastomers—Tension
4. Classification
D 624 Test Method for Tear Strength of Conventional
2 4.1 Constrained—A constrained joint prosthesis is used for
Vulcanized Rubber and Thermoplastic Elastomers
joint replacement and prevents dislocation of the prosthesis in
D 813 Test Method for Rubber-Deterioration—Crack
2 more than one anatomical plane and consists of either a single,
Growth
flexible, across-the-joint component, or more than one compo-
D 1052 Test Method for Measuring Rubber Deterioration—
2 nent linked together or affixed.
Cut Growth Using Ross Flexing Apparatus
D 2240 Test Method for Rubber Property—Durometer
5. Materials and Manufacture
Hardness
5.1 Proper material selection is necessary, but insufficient to
F 67 Specification for an Unalloyed Titanium for Surgical
3 ensure suitable function of a device. All devices conforming to
Implant Application
this specification shall be fabricated from materials with
F 86 Practice for Surface Preparation and Marking of Me-
adequate mechanical strength, durability and biocompatibility.
tallic Surgical Implants
5.2 All elastomeric components shall conform to Specifica-
F 601 Practice for Fluorescent Penetrant Inspection of Me-
tion F 604. Test and evaluation parameters that could be
tallic Surgical Implants
considered for the elastomeric implant materials are Specifi-
F 604 Specification for Silicone Elastomers Used in Medi-
cation F 604, Practice F 748, Test Methods D 813, D 1052,
cal Applications
D 2240, D 412 and D 624. Before implants can be manufac-
F 748 Practice for Selecting Generic Biological Test Meth-
tured from other materials, 5.4 must be comply.
ods for Materials and Devices
5.3 Titanium used as a material of construction for metal
F 981 Practice for Assessment of Compatibility of Bioma-
grommets shall conform to Specification F 67. Metal grommets
terials for Surgical Implants with Respect to Effect of
must match the shape of the implant and not interfere with the
flexible hinge implant function.
This specification is under the jurisdiction of ASTM Committee F04 on
Medical and Surgical Material and Devices and is the direct responsibility of
Subcommittee F04.22 on Arthroplasty.
Current edition approved February 10, 1997. Published June 1997. Available from Superintendent of Documents, U.S. Government Printing
Annual Book of ASTM Standards, Vol 09.01. Office, Washington, DC 20402.
3 6
Annual Book of ASTM Standards, Vol 13.01. Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
Discontinued; See 2000 Annual Book of ASTM Standards, Vol 13.01. 4th Floor, New York, NY 10036.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F1781–97
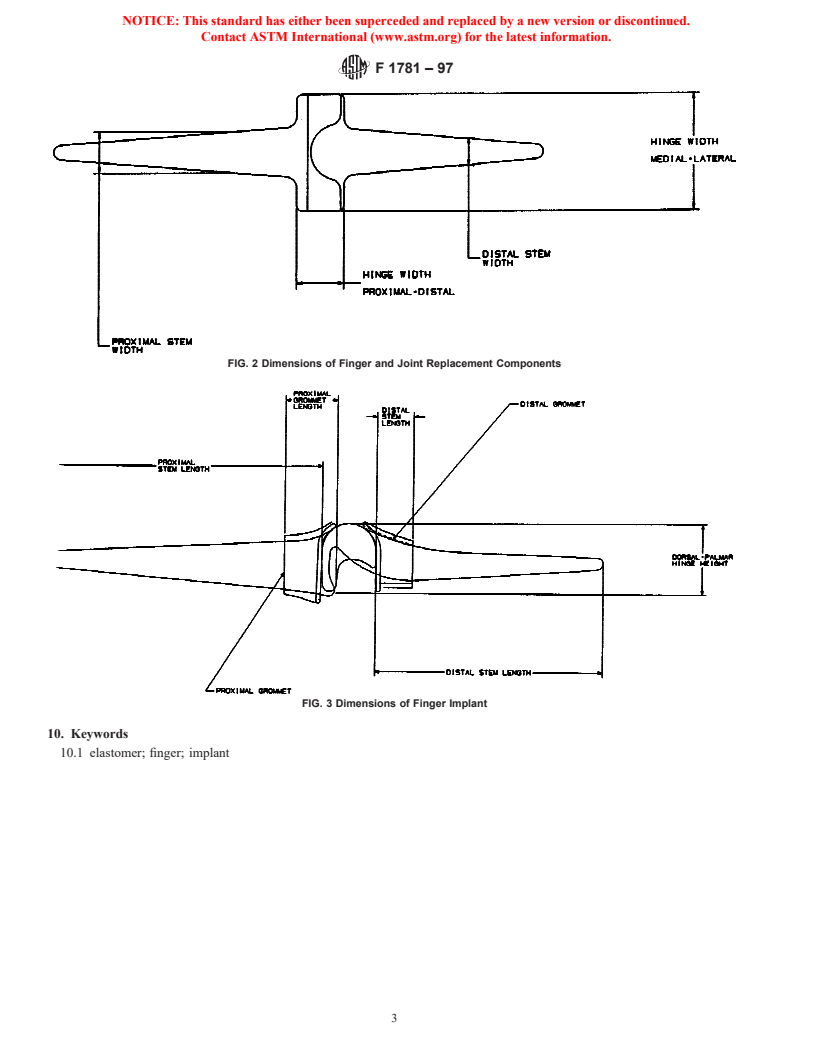
5.4 Biocompatibility—Flexible hinge implants shall be 7.1.1 Distal stem length,
manufactured from the materials listed in 5.2 and 5.3. Before
7.1.2 Proximal stem length,
implants can be manufactured from other materials, their
7.1.3 Hinge width in medial/lateral plane,
biocompatibility must be demonstrated by producing an ac-
7.1.4 Hinge height in dorsal/palmar plane,
ceptable response after testing in accordance with Practices
7.1.5 Distal stem width,
F 748 and F 981, and others (see EN 30993-1) as needed.
7.1.6 Proximal stem width, and
5.5 When appropriate for metallic grommets, fluorescent
7.1.7 Distal-proximal hinge width.
penetrant inspection shall be performed in accordance with
7.2 Dimensions of finger implant with metal grommets shall
Practice F 601.
be reported in labeling (see Fig. 3):
5.6 Design and manufacture will follow 21 CFR Part 820.
7.2.1 Distal stem length,
7.2.2 Proximal stem length,
6. Performance Requirements
7.2.3 Distal grommet length,
6.1 Fatigue Testing—The fatigue characteristics of material
7.2.4 Proximal grommet length, and
from which the elastomeric components are fabricated must be
7.2.5 Hinge height in dorsal/palmar plane.
evaluated according to Test Method D 813. Any test should be
designed to measure fatigue rate (for example, crack growth
8. Finish and Marking
length) as a function of a million(s) cycles.
6.2 Range of Motion of the Device Before Implantation— 8.1 Items conforming to this specification shall be finished
The implant shall be evaluated to determine the maximum
and marked in accordance with Practice F 86 and F 983, where
flexion and extension possible before subluxation occurs or the applicable.
motion is arrested by the implant (elastomer-to-elastomer
8.2 Polymeric Surface Finish—Polymeric Surface Finish
contact within the hinge). These results shall be reported in the shall conform to manufacturer’s documented standards con-
product labeling.
cerning roughness, knit lines, void, bubbles, mold fill, color,
6.3 Guidelines for In-Vitro Laboratory Testing—No ASTM inclusions, and dimensions, when applicable. Descriptions of
standards for testing finger implants have been developed.
these terms can be located in MIL STD 177A.
Laboratory testing that simulates the conditions of use, by a
joint function simulator, is desirable to compare materials and
9. Labeling and Packaging
designs and to provide an indication of clinical performance.
9.1 The maximum range of motion values as determined by
Implant testing shall be done in keeping with the implants
6.2 shall be included in the product labeling. The minimum
intended function, that is, implants intended to partially stabi-
limits for the mechanical properties of the elastomeric materi-
lize or stabilize a joint shall be subjected to the maximum
al(s) used in components shall be included in theproduct
destabilizing force or motion, or both, anticipated in clinical
labeling.
application during flexural testing.
9.2 The dimensions shall be included in the product label-
6.4 Durometer—The hardness of elastomeric components
ing.
shall be measured according to Test Method D 2240.
9.3 The material(s) used for the implant shall be specified in
6.5 The mechanical properties (such as tensile strength,
the package labeling.
percentage elongation, modulus, and tear strength) of the
9.4 The site, orientation (if any), and catalog number if
elastomeric materials used in c
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.