ASTM F2028-14
(Test Method)Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation
Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation
SIGNIFICANCE AND USE
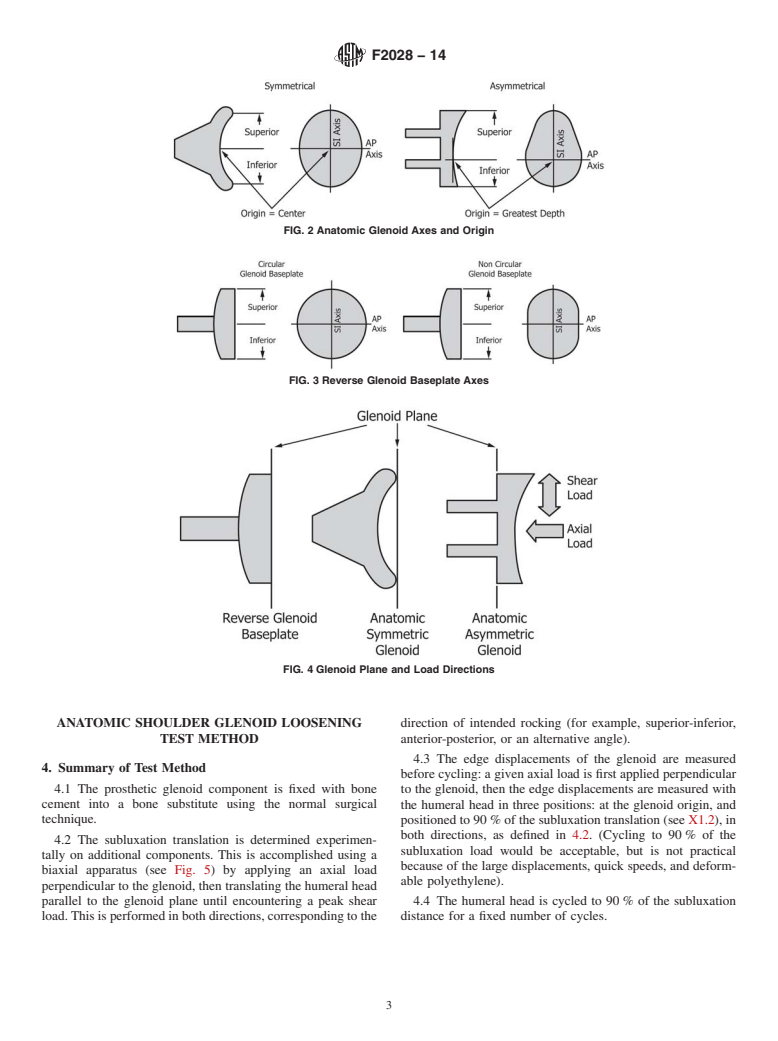
5.1 This test method is intended to investigate the resistance of a glenoid component to loosening. Glenoid loosening is the most common clinical complication in total shoulder arthroplasty (see X1.1). The method assumes that loosening occurs because of edge loading, often called the rocking-horse phenomenon.
5.2 This test method can be used both to detect potential problems and to compare design features. Factors affecting loosening performance include articular geometry, flange geometry, materials, fixation design, bone quality, and surgical technique.
SCOPE
1.1 These test methods measure how much a prosthetic anatomic glenoid component rocks or pivots following cyclic displacement of the humeral head to opposing glenoid rims (for example, superior-inferior or anterior-posterior). Motion is quantified by the tensile displacement opposite each loaded rim after dynamic rocking. Similarly, these test methods measure how much a prosthetic reverse glenoid component rocks or pivots following cyclic articulation with a mating humeral liner. Motion is quantified by the magnitude of displacement measured before and after cyclic loading.
1.2 The same setup can be used to test the locking mechanisms of modular glenoid components, for example, disassociation of both anatomic and reverse shoulder components.
1.3 These test methods cover shoulder replacement designs with monolithic or modular glenoid components for cemented fixation as well as reverse glenoid components for uncemented fixation.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2028 − 14
Standard Test Methods for
Dynamic Evaluation of Glenoid Loosening or
1
Disassociation
This standard is issued under the fixed designation F2028; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope F1839 Specification for Rigid Polyurethane Foam for Use as
a Standard Material for Testing Orthopaedic Devices and
1.1 These test methods measure how much a prosthetic
Instruments
anatomic glenoid component rocks or pivots following cyclic
displacementofthehumeralheadtoopposingglenoidrims(for
3. Terminology
example, superior-inferior or anterior-posterior). Motion is
quantifiedbythetensiledisplacementoppositeeachloadedrim
3.1 Definitions:
after dynamic rocking. Similarly, these test methods measure
3.1.1 anatomic total shoulder arthroplasty, n—shoulder im-
how much a prosthetic reverse glenoid component rocks or
plant that has a concave glenoid component and a convex
pivots following cyclic articulation with a mating humeral
humeral component design.
liner. Motion is quantified by the magnitude of displacement
3.1.1.1 anatomic glenoid, n—the concave prosthetic portion
measured before and after cyclic loading.
that replaces the glenoid fossa of the scapula and articulates
1.2 The same setup can be used to test the locking mecha-
with a convex prosthetic replacement of the humeral head in
nisms of modular glenoid components, for example, disasso-
anatomic total shoulder arthroplasty applications. It may con-
ciation of both anatomic and reverse shoulder components.
sistofoneormorecomponentsfromoneormorematerials,for
example, either all-polyethylene or a metal baseplate with a
1.3 These test methods cover shoulder replacement designs
polymeric insert.
with monolithic or modular glenoid components for cemented
fixation as well as reverse glenoid components for uncemented
3.1.1.2 humeral head, n—the convex prosthetic portion that
fixation.
replaces the proximal humerus or humeral head and articulates
with the natural glenoid fossa or an anatomic prosthetic
1.4 The values stated in SI units are to be regarded as
replacement.
standard. No other units of measurement are included in this
standard.
3.1.2 reverse total shoulder arthroplasty, n—shoulder im-
plants that have a convex glenoid component and a concave
1.5 This standard does not purport to address all of the
humeral component design.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
3.1.2.1 glenoid baseplate, n—the nonarticular portion of the
priate safety and health practices and determine the applica-
reverse glenoid component that modularly connects to the
bility of regulatory limitations prior to use.
glenosphere and is usually fixed to the glenoid fossa of the
scapula using bone screws without the use of cement.
2. Referenced Documents
3.1.2.2 glenosphere, n—the convex prosthetic articular por-
2
2.1 ASTM Standards:
tion of the reverse glenoid component that articulates with the
E4 Practices for Force Verification of Testing Machines
concave prosthetic replacement of the proximal humerus or
F1378 Specification for Shoulder Prostheses
humeral head (for example, the humeral liner).
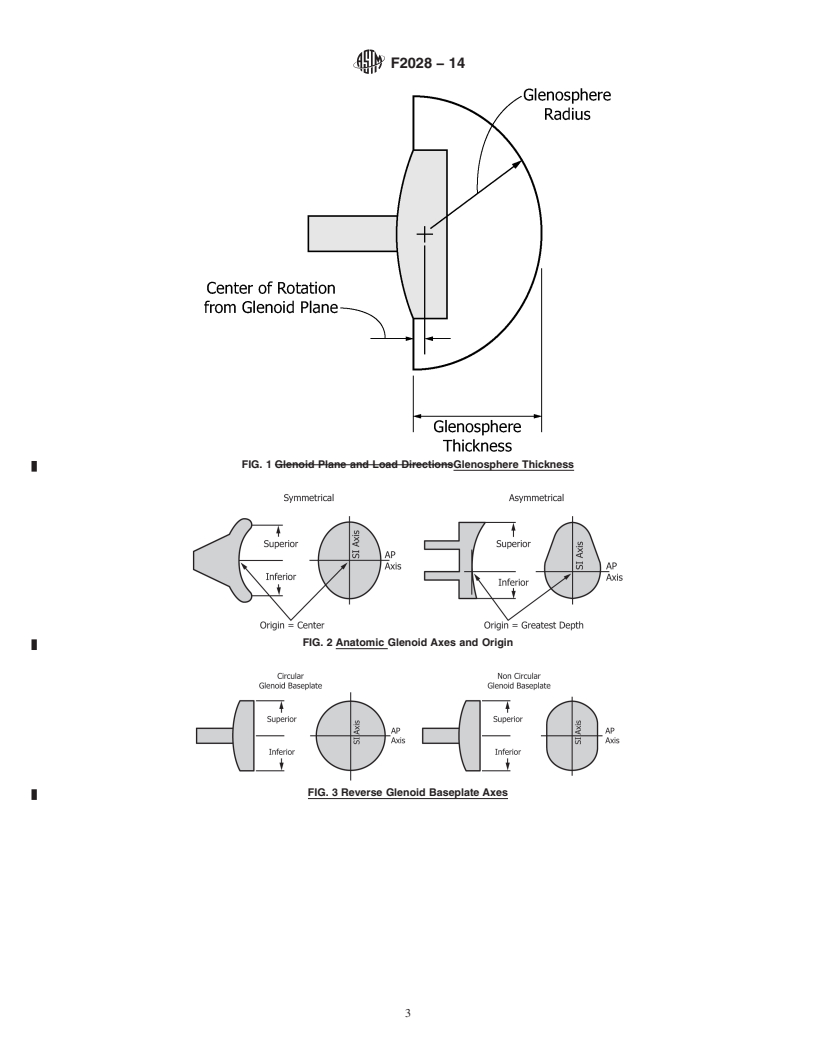
3.1.2.3 glenosphere thickness, n—theheightofthetruncated
section of the sphere which composes the glenosphere. Note
1
These test methods are under the jurisdiction of ASTM Committee F04 on
thatthedifferencebetweentheglenospherearticularradiusand
Medical and Surgical Materials and Devices and are the direct responsibility of
Subcommittee F04.22 on Arthroplasty.
thickness defines the medial/lateral position of the glenoid
Current edition approved March 1, 2014. Published July 2014. Originally
center of rotation (see Fig. 1).The glenosphere thickness could
ε1
approved in 2000. Last previous edition approved in 2012 as F2028 – 08(2012) .
also be affected by the geometric relation between the gleno-
DOI: 10.1520/F2028-14.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or sphere and the glenoid baseplate.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3.1.2.4 humeral liner, n—the concave prosthetic portion of
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. the reverse humeral component that replaces the proximal
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2028 − 14
humerus or humeral head and articulates with the convex 3.1.6 glenoid plane (see X1.9),n—in symm
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
´1
Designation: F2028 − 08 (Reapproved 2012) F2028 − 14
Standard Test Methods for
Dynamic Evaluation of Glenoid Loosening or
1
Disassociation
This standard is issued under the fixed designation F2028; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1
ε NOTE—Units information was editorially corrected in January 2013.
1. Scope
1.1 These test methods measure how much a prosthetic anatomic glenoid component rocks or pivots following cyclic
displacement of the humeral head to opposing glenoid rims (for example, superior-inferior or anterior-posterior). Performanc-
eMotion is judgedquantified by the tensile displacement opposite each loaded rim after dynamic rocking. Similarly, these test
methods measure how much a prosthetic reverse glenoid component rocks or pivots following cyclic articulation with a mating
humeral liner. Motion is quantified by the magnitude of displacement measured before and after cyclic loading.
1.2 The same setup can be used to test the locking mechanismmechanisms of modular glenoid components, for example, for
disassociation. disassociation of both anatomic and reverse shoulder components.
1.3 These test methods cover shoulder replacement designs with monolithic or modular glenoid components for cemented
fixation as well as reverse glenoid components for uncemented fixation.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ASTM Standards:
E4 Practices for Force Verification of Testing Machines
F1378 Specification for Shoulder Prostheses
F1839 Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and
Instruments
3. Terminology
3.1 Definitions:
3.1.1 glenoid—anatomic total shoulder arthroplasty, n—the prosthetic portion that replaces the glenoid fossa of the scapula and
articulates with a prosthetic replacement of the humeral head. It may consist of one or more components from one or more
materials, for example, either all polyethylene or a metal baseplate with a polymeric insert.shoulder implant that has a concave
glenoid component and a convex humeral component design.
3.1.1.1 anatomic glenoid, n—the concave prosthetic portion that replaces the glenoid fossa of the scapula and articulates with
a convex prosthetic replacement of the humeral head in anatomic total shoulder arthroplasty applications. It may consist of one
or more components from one or more materials, for example, either all-polyethylene or a metal baseplate with a polymeric insert.
3.1.1.2 humeral head, n—the convex prosthetic portion that replaces the proximal humerus or humeral head and articulates with
the natural glenoid fossa or an anatomic prosthetic replacement.
1
These test methods are under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and are the direct responsibility of Subcommittee
F04.22 on Arthroplasty.
Current edition approved Dec. 15, 2012March 1, 2014. Published January 2013July 2014. Originally approved in 2000. Last previous edition approved in 20082012 as
ε1
F2028 – 08.F2028 – 08(2012) . DOI: 10.1520/F2028-08R12E01.10.1520/F2028-14.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’sstandard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2028 − 14
3.1.2 humeral head—reverse total shoulder arthroplasty, n—the prosthetic portion that replaces the proximal humerus or
humeral head and articulates with the natural glenoid fossa or a prosthetic replacement.shoulder implants that have a convex
glenoid component and a concave humeral component design.
3.1.2.1 glenoid baseplate, n—the nonarticular portion of the reverse glenoid component that
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.