ASTM F3295-18
(Guide)Standard Guide for Impingement Testing of Total Disc Prostheses
Standard Guide for Impingement Testing of Total Disc Prostheses
SIGNIFICANCE AND USE
5.1 This guide can be used to develop test parameters for evaluating fatigue and wear behavior of IVD prostheses under impingement loading. It must be recognized, however, that there are likely many possible impingement conditions for a given IVD prosthesis.
5.2 The user should attempt to determine the clinically relevant and geometrically possible impingement conditions and dictated by the design and impingement wear test parameters that may result in wear and fatigue damage for the IVD prosthesis. The user should also attempt to select the device size which will represent a worst case for the impingement conditions and parameters selected.
5.3 The user should reference and utilize existing sources of information to identify the impingement test parameters that produce the clinically relevant impingement wear and damage for their IVD prosthesis. Prior clinical experience with the device design may aid in the development of impingement test parameters through analysis of device retrievals and radiographs. However, prior clinical experience with the IVD being tested should not be considered as a prerequisite for performing impingement testing.
5.4 This guide details a three-step process for assessing device impingement under a selected set of conditions:
5.4.1 The user selects previously identified impingement conditions, one at a time, or clinically observed conditions.
5.4.2 The user selects the worst-case size of device to apply the selected conditions.
5.4.3 Solid modeling and the quasistatic test method should be employed to assess the impingement condition and determine the impingement test parameters – most importantly, the angular displacement limits to be used in the impingement wear test.
5.4.4 The impingement wear test is then conducted using the impingement test parameters.
5.5 This guide serves to evaluate devices with various designs, materials (i.e., metal-on-metal versus polymer-on-polymer), and stiffness in the impingement reg...
SCOPE
1.1 This standard is intended to provide guidance on the evaluation of wear and fatigue characteristics of total disc prostheses under cyclic impingement conditions.
1.2 This guide describes impingement testing of devices with articulating components. The user is cautioned that the methods described herein are intended to produce an impingement condition which may or may not be indicative of clinical performance and which may or may not be consistent with the intended use of the device, and that this should be considered when interpreting the data. Clinically, total disc prostheses should always be implanted per labeling and the manufacturer’s instructions for use.
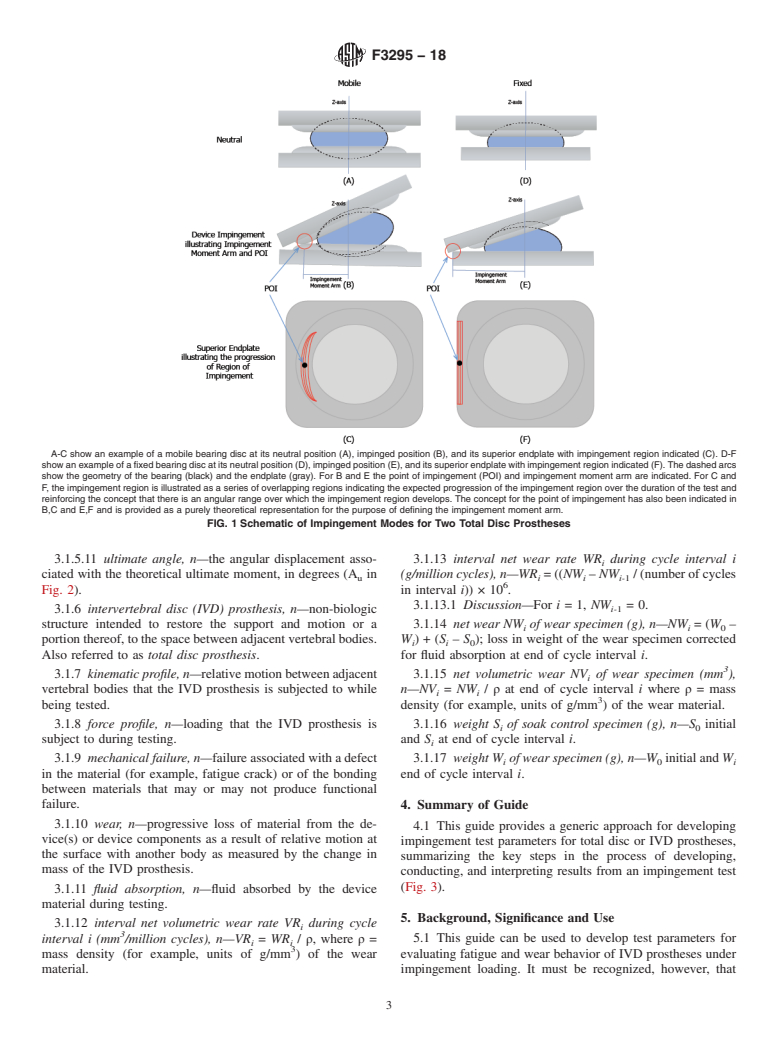
1.3 Impingement has been observed in retrievals among several total disc prosthesis designs; however, impingement is not necessarily associated with device or clinical failure. It is the intent of this guide to investigate possible impingement-induced wear and mechanical failure modes associated with device design, as well as potential mechanical failure modes associated with clinical events such as subsidence, malpositioning, and improper implant sizing. Note that mechanical failure may or may not be associated with functional failure.
1.4 It is recommended that the user define the bearing and non-bearing features of the intervertebral disc (IVD) prosthesis and evaluate the performance of the IVD prosthesis under Mode 1 wear by using Guide F2423 or ISO 18192-1 prior to use of this guide. This standard is not intended to provide guidance on Mode I testing.
1.5 The goal of this guide is to evaluate impingement in IVD prostheses regardless of the intended region of the spine (cervical or lumbar), material or material combinations (ceramic, metal, polymer), and bearing type (fixed or mobile).
1.6 It is the intent of this guide to enable comparison of IVD prostheses with regard to wear and fatigue characteristics when tested under the specified cond...
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F3295 − 18
Standard Guide for
1
Impingement Testing of Total Disc Prostheses
This standard is issued under the fixed designation F3295; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.7 The values stated in SI units are to be regarded as the
standard with the exception of angular measurements which
1.1 This standard is intended to provide guidance on the
should be reported in degrees.
evaluation of wear and fatigue characteristics of total disc
1.8 The use of this standard may involve the operation of
prostheses under cyclic impingement conditions.
potentially hazardous equipment. This standard does not pur-
1.2 This guide describes impingement testing of devices
port to address all of the safety concerns, if any, associated
with articulating components. The user is cautioned that the
with its use. It is the responsibility of the user of this standard
methods described herein are intended to produce an impinge-
to establish appropriate safety, health, and environmental
ment condition which may or may not be indicative of clinical
practices and determine the applicability of regulatory limita-
performance and which may or may not be consistent with the
tions prior to use.
intended use of the device, and that this should be considered
1.9 This international standard was developed in accor-
when interpreting the data. Clinically, total disc prostheses
dance with internationally recognized principles on standard-
should always be implanted per labeling and the manufactur-
ization established in the Decision on Principles for the
er’s instructions for use.
Development of International Standards, Guides and Recom-
1.3 Impingement has been observed in retrievals among
mendations issued by the World Trade Organization Technical
several total disc prosthesis designs; however, impingement is
Barriers to Trade (TBT) Committee.
not necessarily associated with device or clinical failure. It is
2. Referenced Documents
the intent of this guide to investigate possible impingement-
2
induced wear and mechanical failure modes associated with
2.1 ASTM Standards:
device design, as well as potential mechanical failure modes
E4Practices for Force Verification of Testing Machines
associated with clinical events such as subsidence,
E1402Guide for Sampling Design
malpositioning, and improper implant sizing. Note that me-
E1488GuideforStatisticalProcedurestoUseinDeveloping
chanical failure may or may not be associated with functional
and Applying Test Methods
failure.
F561 Practice for Retrieval and Analysis of Medical
Devices, and Associated Tissues and Fluids
1.4 It is recommended that the user define the bearing and
F1714GuideforGravimetricWearAssessmentofProsthetic
non-bearingfeaturesoftheintervertebraldisc(IVD)prosthesis
Hip Designs in Simulator Devices
and evaluate the performance of the IVD prosthesis under
F1877Practice for Characterization of Particles
Mode 1 wear by using Guide F2423 or ISO 18192-1 prior to
F2423Guide for Functional, Kinematic, and Wear Assess-
use of this guide. This standard is not intended to provide
ment of Total Disc Prostheses
guidance on Mode I testing.
3
2.2 ISO Standard:
1.5 The goal of this guide is to evaluate impingement in
ISO 18192–1Implants for surgery—Wear of total interver-
IVD prostheses regardless of the intended region of the spine
tebral spinal disc prostheses—Part 1: Loading and dis-
(cervical or lumbar), material or material combinations
placement parameters for wear testing and corresponding
(ceramic, metal, polymer), and bearing type (fixed or mobile).
environmental
1.6 ItistheintentofthisguidetoenablecomparisonofIVD
3. Terminology
prostheseswithregardtowearandfatiguecharacteristicswhen
tested under the specified conditions.
3.1 Definitions of Terms Specific to This Standard:
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
1
This guide is under the jurisdiction ofASTM Committee F04 on Medical and contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Surgical Materials and Devices and is the direct responsibility of Subcommittee Standards volume information, refer to the standard’s Document Summary page on
F04.25 on Spinal Devices. the ASTM website.
3
Current edition approved July 1, 2018. Published August 2018. DOI: 10.1520/ Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
F3295–18. 4th Floor, New York, NY 10036, http://www.ansi.or
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.