ASTM D4953-99a
(Test Method)Standard Test Method for Vapor Pressure of Gasoline and Gasoline-Oxygenate Blends (Dry Method)
Standard Test Method for Vapor Pressure of Gasoline and Gasoline-Oxygenate Blends (Dry Method)
SCOPE
1.1 This test method, a modification of Test Method D323 (Reid Method), provides two procedures to determine the vapor pressure (Note 1) of gasoline and gasoline-oxygenate blends. This test method is applicable to gasolines and gasoline-oxygenate blends with a vapor pressure range from 35 to 100 kPa (5 to 15 psi) (see Note 2). Note 1-Because the external atmospheric pressure is counteracted by the atmospheric pressure initially present in the air chamber, this vapor pressure is an absolute pressure at 37.8°C (100°F) in kilopascals (pounds-force per square inch). This vapor pressure differs from the true vapor pressure of the sample due to some small vaporization of the sample and air in the confined space. Note 2-Vapor pressure of gasoline or gasoline-oxygenate blends below 35 kPa (5 psi) or greater than 100 kPa (15 psi) can be determined with this test method but the Precision and Bias as described in Section 10 do not apply. For materials with a vapor pressure greater than 100 kPa (15 psi), use a 0 to 200 kPa (0 to 30 psi) gage as specified in the Annex of Test Method D323.
1.3 The values stated in acceptable metric units are standard. The values given in parentheses are provided for information purposes only.
1.4 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautions are given in 6.5, and Notes 4, 6, 7, A1.1, and A1.2.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation: D 4953 – 99a
Standard Test Method for
Vapor Pressure of Gasoline and Gasoline-Oxygenate Blends
(Dry Method)
This standard is issued under the fixed designation D 4953; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope ucts (Reid Method)
D 4057 Practice for Manual Sampling of Petroleum and
1.1 This test method, a modification of Test Method D 323
Petroleum Products
(Reid Method), provides two procedures to determine the
E 1 Specification for ASTM Thermometers
vapor pressure (Note 1) of gasoline and gasoline-oxygenate
blends. This test method is applicable to gasolines and
3. Summary of Test Method
gasoline-oxygenateblendswithavaporpressurerangefrom35
3.1 The liquid chamber of the vapor pressure apparatus is
to 100 kPa (5 to 15 psi) (see Note 2).
filled with the chilled sample and connected to the vapor
NOTE 1—Because the external atmospheric pressure is counteracted by
chamber at 37.8°C (100°F). The apparatus is immersed in a
the atmospheric pressure initially present in the air chamber, this vapor
bath at 37.8°C (100°F) until a constant pressure is observed.
pressure is an absolute pressure at 37.8°C (100°F) in kilopascals (pounds-
The pressure reading, suitably corrected, is reported as the
force per square inch). This vapor pressure differs from the true vapor
vapor pressure.
pressure of the sample due to some small vaporization of the sample and
3.2 Procedure A utilizes the same apparatus and essentially
air in the confined space.
NOTE 2—Vapor pressure of gasoline or gasoline-oxygenate blends
the same procedure as Test Method D 323 with the exception
below 35 kPa (5 psi) or greater than 100 kPa (15 psi) can be determined
that the interior surfaces of the liquid and vapor chambers are
with this test method but the Precision and Bias as described in Section 10
maintained completely free of water. Procedure B utilizes a
do not apply. For materials with a vapor pressure greater than 100 kPa (15
semi-automatic apparatus with the liquid and vapor chambers
psi), usea0to200kPa(0to30 psi) gage as specified in theAnnex ofTest
identical in volume to those in Procedure A. The apparatus is
Method D 323.
suspended in a horizontal bath and rotated while attaining
1.2 Somegasoline-oxygenateblendsmayshowahazewhen
equilibrium. Either a Bourdon gage or pressure transducer can
cooled to 0 to 1°C. If a haze is observed in 8.4, it shall be
be used with this procedure. The interior surfaces of the liquid
indicated in the reporting of results. The precision and bias
and vapor chambers are maintained free of water.
statements for hazy samples have not been determined (see
Note 10).
4. Significance and Use
1.3 The values stated in acceptable metric units are stan-
4.1 Vapor pressure is an important physical property of
dard. The values given in parentheses are provided for infor-
liquid spark-ignition engine fuels. It provides an indication of
mation purposes only.
how a fuel will perform under different operating conditions.
1.4 This standard does not purport to address all of the
For example, vapor pressure is a factor in determining whether
safety concerns, if any, associated with its use. It is the
a fuel will cause vapor lock at high ambient temperature or at
responsibility of the user of this standard to establish appro-
high altitude, or will provide easy starting at low ambient
priate safety and health practices and determine the applica-
temperature.
bility of regulatory limitations prior to use. Specific precau-
4.2 Petroleum product specifications generally include va-
tions are given in 6.5, and Note 5, Note 7, Note 8, Note A1.1,
por pressure limits to ensure products of suitable volatility
and Note A1.2.
performance.
2. Referenced Documents
NOTE 3—Vapor pressure of fuels is regulated by various government
agencies.
2.1 ASTM Standards:
D 323 Test Method for Vapor Pressure of Petroleum Prod-
5. Apparatus
5.1 TheapparatusforProcedureAisdescribedinAnnexA1.
This test method is under the jurisdiction of Committee D-2 on Petroleum
Products and Lubricants and is the direct responsibility of Subcommittee D02.08 on
Volatility. Annual Book of ASTM Standards, Vol 05.01.
Current edition approved April 10, 1999. Published June 1999. Originally Annual Book of ASTM Standards, Vol 05.02.
published as D 4953 – 89. Last previous edition D 4953 – 99. Annual Book of ASTM Standards, Vol 14.03.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 4953
after being immersed and withdrawn from the sample. To confirm the
5.2 Theessentialdimensionsandrequirementsfortheliquid
sample volume, insert the dipstick into the sample container so that it
and vapor chamber for Procedure B are identical with those for
touches the bottom of the container at a perpendicular angle, before
Procedure A and described in Annex A1. External fittings and
removing the dipstick. For transparent containers, using a marked ruler or
features will vary depending on whether a gage or transducer
by comparing the sample container to a like container which has the 70
is used and the provision for rotating the apparatus in the bath.
and 80 % levels clearly marked, has been found suitable.
Details of a commercially available unit are shown in Annex
7.2.1 Discard the sample if its volume is less than 70 % of
A2.
the container capacity.
7.2.2 If the container is more than 80 % full, pour out
6. Handling of Test Samples
enough sample to bring the container contents within 70 to
6.1 This section applies to both Procedure A and B.
80 % range. Under no circumstance return any of the poured
6.2 The extreme sensitivity of vapor pressure measurements
out sample to the container.
to losses through evaporation is such as to require the utmost
7.2.3 Reseal the container, if necessary, and return the
precaution and the most meticulous care in handling of
sample container to the cooling bath.
samples.
7.3 Air Saturation of the Sample in Sample Container:
6.3 Sampling shall be done in accordance with the Reid
7.3.1 Transparent Containers Only—Since 7.2 does not
Vapor Pressure section (10.3) of Practice D 4057 except for
requirethatthesamplebeopenedtoverifythesamplecapacity,
fuels containing oxygenates where the Water Displacement
it is necessary to unseal the cap momentarily before resealing
Procedure section (10.3.1.8) of D 4057 must not be used.
it, so that the samples in transparent containers are treated the
6.4 Sample Container Size:
same as samples in non-transparent containers.
6.4.1 The size of the sample container from which the vapor
7.3.2 With the sample again at a temperature of 0 to 1°C,
pressure sample is taken shall be 1 L (1 qt). It shall be 70 to
take the container from the cooling bath or refrigerator, wipe it
80 % filled with sample.
dry with an absorbent material, remove the cap momentarily,
6.4.2 The present precision statement has been derived
taking care that no water enters, reseal, and shake vigorously.
using samples in 1-L (1-qt) containers. Samples taken in
Return it to the cooling bath or refrigerator for a minimum of
containers of other sizes as prescribed in Practice D 4057 can
2 min.
be used if it is recognized that the precision can be affected. In
7.3.3 Repeat 7.3.2 twice more. Return the sample to the
the case of referee testing the 1-L(1-qt) sample container shall
cooling bath until the beginning of the procedure.
be mandatory.
7.4 Preparation of Liquid Chamber:
6.5 Hazard:
7.4.1 Place the stoppered or closed liquid chamber and the
6.5.1 The vapor pressure determination shall be the first test
sample transfer tube in a refrigerator or cooling bath for
withdrawnfromthesamplecontainer.Theremainingsamplein
sufficient time to allow the chamber and the transfer tube to
the container cannot be used for a second vapor pressure
reach a temperature of 0 to 1°C (32 to 34°F). Keep the liquid
determination. If necessary, obtain a new sample.
chamber upright and not immersed over the top of the coupling
6.5.2 Samples shall be protected from excessive heat prior
threads.
to testing.
6.5.3 Samples in leaky containers shall not be tested. NOTE 5—Caution: The transfer connection must be kept completely
dry during cooling. This can be accomplished by placing the transfer
Discard and obtain a new sample.
connection in a water tight plastic bag.
6.6 Sample Handling Temperature—In all cases, the sample
container and contents shall be cooled to 0 to 1°C (32 to 34°F) 7.5 Preparation of the Vapor Chamber
before the container is opened. Sufficient time to reach this 7.5.1 Connect the gage or pressure transducer to the vapor
temperature shall be assured by direct measurement of the chamber and make a water tight closure of the lower opening
temperature of a similar liquid in a like container placed in the of the chamber where the liquid chamber attaches. Make sure
cooling bath at the same time as the sample. See A1.3.1. that the vent hole in the vapor chamber connection is also
securely closed.
7. Preparation of Apparatus
NOTE 6—For someTest Method D 323 apparatus, a Number 6.5 rubber
7.1 This section applies to both Procedure A and Procedure
stopper has been found satisfactory. For the horizontal or Herzog
B.
apparatus, a Number 3 rubber stopper and a Number 000 cork in the vent
7.2 Verification of Sample Container Filling—With the
hole is satisfactory.Another procedure is to attach a spare liquid chamber
sampleatatemperatureof0to1°C,takethecontainerfromthe to the vapor chamber during the conditioning period.Athird alternative is
to utilize a cap threaded to match the threads of the vapor chamber.
cooling bath or refrigerator and wipe dry with absorbent
Several apparatus manufacturers have indicated the intention to supply
material. If the container is not transparent, unseal it, and using
such caps for equipment. In any procedure used, the interior surfaces of
a suitable gage, confirm that the sample volume equals 70 to
the vapor pressure apparatus and the sample must be kept completely free
80 % of the container capacity (see Note 4). If the sample is
of water.
contained in a transparent glass container, verify that the
NOTE 7—Caution: Making a water tight closure of both the liquid and
container is 70 to 80 % full by suitable means (see Note 4).
vapor chambers is extremely important. For some samples containing
oxygenated compounds, contact with water can cause phase separation
NOTE 4—For non-transparent containers, one way to confirm that the
and invalidate results.
sample volume equals 70 to 80 % of the container capacity is to use a
7.5.2 Immerse the vapor chamber in a water bath main-
dipstick that has been pre-marked to indicate the 70 and 80 % container
capacities. The dipstick should be of such material that it shows wetting tained at 37.8 6 0.1°C (100 6 0.2°F) for not less than 20 min.
D 4953
The top of the vapor chamber must be at least 25 mm (1 in.) it vigorously eight times lengthwise. With the gage end up,
below the surface of the water (ProcedureA). (In Procedure B immerse the assembled apparatus in the bath, maintained at
the vapor chamber lies horizontally, completely immersed in 37.8 6 0.1°C (100 6 0.2°F), in an inclined position so that the
the water bath.) Do not remove the vapor chamber from the connectionoftheliquidandvaporchambersisbelowthewater
water bath until the liquid chamber has been filled with sample level. Carefully examine for leaks. If no leaks are observed,
as described in 8.1. further immerse the apparatus to at least 25 mm (1 in.) above
the top of the vapor chamber. Observe the apparatus for leaks
8. Procedure
throughout the test and discard the test at anytime a leak is
detected.
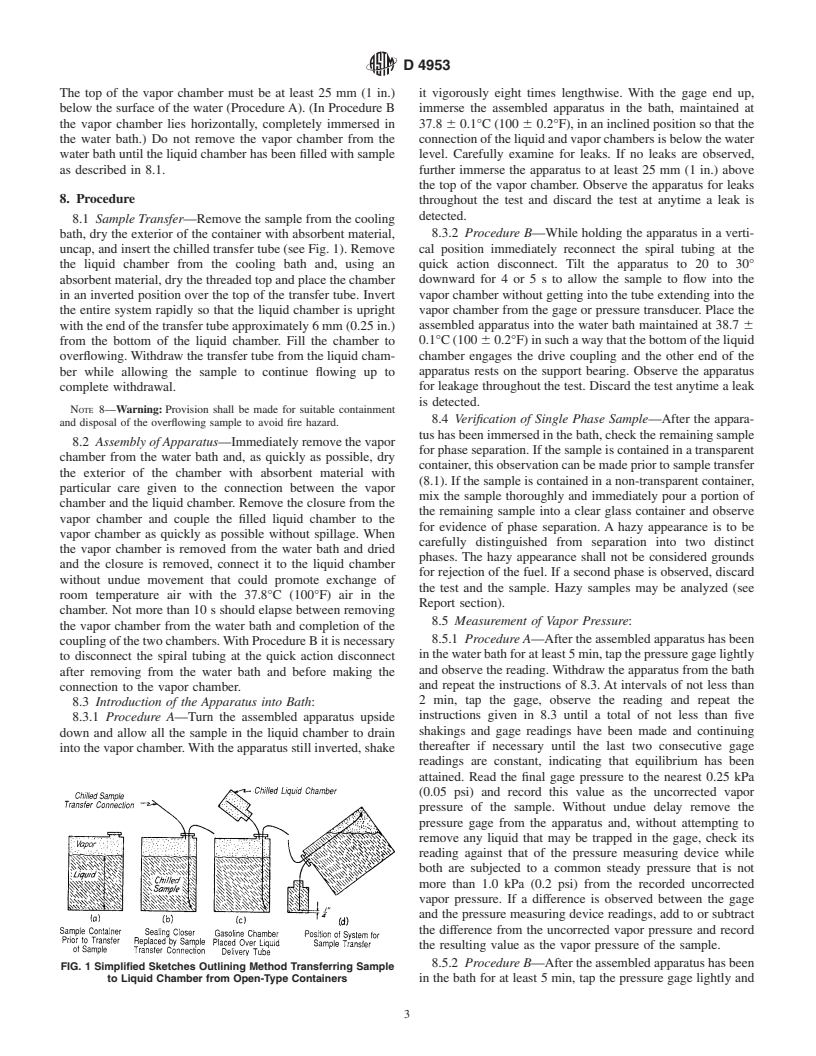
8.1 Sample Transfer—Remove the sample from the cooling
8.3.2 Procedure B—While holding the apparatus in a verti-
bath, dry the exterior of the container with absorbent material,
uncap, and insert the chilled transfer tube (see Fig. 1). Remove cal position immediately reconnect the spiral tubing at the
quick action disconnect. Tilt the apparatus to 20 to 30°
the liquid chamber from the cooling bath and, using an
absorbent material, dry the threaded top and place the chamber downward for 4 or5sto allow the sample to flow into the
vapor chamber without getting into the tube extending into the
in an inverted position over the top of the transfer tube. Invert
the entire system rapidly so that the liquid chamber is upright vapor chamber from the gage or pressure transducer. Place the
assembled apparatus into the water bath maintained at 38.7 6
with the end of the transfer tube approximately 6 mm (0.25 in.)
from the bottom of the liquid chamber. Fill the chamber to 0.1°C(100 60.2°F)insuchawaythatthebottomoftheliquid
chamber engages the drive coupling and the other end of the
overflowing. Withdraw the transfer tube from the liquid cham-
ber while allowing the sample to continue flowing up to apparatus rests on the support bearing. Observe the apparatus
for leakage throughout the test. Discard the test anytime a leak
complete withdrawal.
is detected.
NOTE 8—Warning: Provision shall be made for suitable containment
8.4 Verification of Single Phase Sample—After the appara-
and disposal of the overflowing sample to avoid fire hazard.
tus has been immersed in the bath, check the remaining sample
8.2 Assembly of Apparatus—Immediately remove the vapor
for phase separation. If the sample is contained in a transparent
chamber from the water bath and, as quickly as possible, dry
container,thisobservationcanbemadepriortosampletransfer
the exterior of the chamber with absorbent material with
(8.1). If the sample is contained in a non-transparent container,
particular care given to the connection between the vapor
mix the sample thoroughly and immediately pour a portion of
chamber and the liquid chamber. Remove the closure from the
the remaining sample into a clear glass container and observe
vapor chamber and couple the filled liquid chamber to the
for evidence of phase separation. A hazy appearance is to be
vapor chamber as quickly as possible without spillage. When
carefully distinguished from separation into two distinct
the vapor chamber is removed from the water bath and dried
phases. The hazy appearance shall not be considered grounds
and the closure is removed, connect it to the liquid chamber
for rejection of the fuel. If a second phase is observed, discard
without undue movement that could promote exchange of
the test and the sample. Hazy samples m
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.