ASTM F1357-99
(Specification)Standard Specification for Articulating Total Wrist Implants
Standard Specification for Articulating Total Wrist Implants
SCOPE
1.1 This specification describes total wrist implants, including solid ceramic implants, used to provide functioning articulation by employing radial carpal components.
1.2 This specification excludes those implants with ceramic-coated or porous-coated surfaces, one piece elastomeric implants, and those devices used for custom applications.
1.3 The values stated in SI units are standard. The English values in parentheses are for information only.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 1357 – 99
Standard Specification for
Articulating Total Wrist Implants
This standard is issued under the fixed designation F 1357; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope ethylene Powder and Fabricated Form for Surgical Im-
plants
1.1 This specification describes total wrist implants, includ-
F 748 Practice for Selecting Generic Biological Test Meth-
ing solid ceramic implants, used to provide functioning articu-
ods for Materials and Devices
lation by employing radial carpal components.
F 799 Specification for Thermomechanically Processed
1.2 This specification excludes those implants with ceramic-
Cobalt-Chromium-Molybdenum Alloy for Surgical Im-
coated or porous-coated surfaces, one piece elastomeric im-
plants
plants (with or without grommets), and those devices used for
F 981 Practice for Assessment of Compatibility of Bio-
custom applications.
Materials (Non-Porous) for Surgical Implants with Respect
1.3 The values stated in SI units are standard. The English
to Effect of Materials on Muscle and Bone
values in parentheses are for information only.
F 983 Practice for Permanent Marking of Orthopaedic Im-
2. Referenced Documents plant Components
F 1108 Specification for Ti6A14V Alloy Castings for Sur-
2.1 ASTM Standards:
gical Implants
F 67 Specification for an Unalloyed Titanium for Surgical
F 1537 Specification for Wrought Cobalt-28 Chromium-6
Implant Application
Molybdenum Alloy for Surgical Implants
F 75 Specification for Cast Cobalt-Chromium-Molybdenum
Alloy for Surgical Implant Applications
3. Terminology
F 86 Practice for Surface Preparation and Marking of Me-
3.1 Definitions:
tallic Surgical Implants
3.1.1 carpal component—articulating member inserted into
F 90 Specification for Wrought Cobalt-Chromium-
or through the carpal bones.
Tungsten-Nickel Alloy for Surgical Implant Applications
3.1.2 radial component—articulating member inserted into
F 136 Specification for Wrought Titanium 6A1-4V ELI
the radius for articulation with the carpal component.
Alloy for Surgical Implant Applications
3.1.3 total wrist replacement—prosthetic parts substituted
F 562 Specification for Wrought Cobalt-Nickel-Chromium-
for the native opposing radial and carpal articulating surfaces.
Molybdenum Alloy for Surgical Implant Applications
F 563 Specification for Wrought Cobalt-Nickel-Chromium-
4. Classification
Molybdenum-Tungsten-Iron Alloy for Surgical Implant
2 4.1 Constrained—A constrained joint prosthesis is used for
Applications
joint replacement and prevents dislocation of the prosthesis in
F 601 Practice for Fluorescent Penetrant Inspection of Me-
2 more than one anatomical plane and consists of either a single,
tallic Surgical Implants
flexible, across-the-joint component, or more than one compo-
F 603 Specification for High-Purity Dense Aluminum Ox-
2 nent linked together or affined.
ide for Surgical Implant Application
4.2 Partially Constrained—A semi-constrained joint pros-
F 629 Practice for Radiography of Cast Metallic Surgical
thesis is used for partial or total joint replacement and limits
Implants
translation and rotation of the prosthesis in one or more planes
F 648 Specification for Ultra-High-Molecular-Weight Poly-
via the geometry of its articulating surfaces. It has no across-
the-joint linkages.
4.3 Unconstrained—An unconstrained joint prosthesis is
This specification is under the jurisdiction of ASTM Committee F-4 on Medical
and Surgical Materials and Devicesand is the direct responsibility of Subcommittee
used for partial or total joint replacement and restricts mini-
F04.22 on Anthroplasty.
mally prosthesis movement in one or more planes. Its compo-
Current edition approved November 10, 1999. Published December 1999.Origi-
nents have no across-the-joint linkages.
nally published as F 1357 - 91. Last previous edition F 1357 - 91 (1997).
Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F 1357
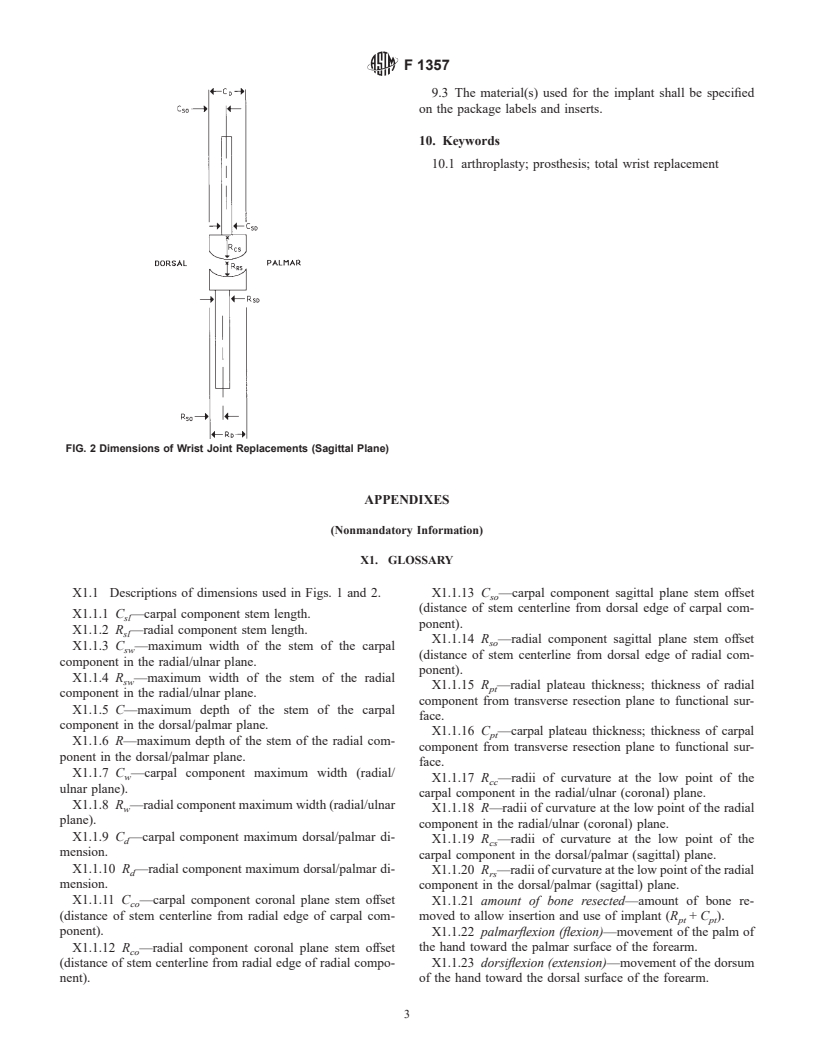
5. Materials and Manufacture 7. Dimensions
7.1 Dimensions of wrist joint replacement components shall
5.1 Proper material selection is necessary, but insufficient to
be as designated in Figs. 1 and 2.
ensure suitable function of a device.
5.2 All metal implant components shall conform to one of
8. Finish and Marking
the following specifications for implant materials: Specifica-
8.1 Items conforming to this specification shall be finished
tion F 67, F 75, F 90, F 136, F 562, F 563 (nonbearing use
and marked in accordance with Practice F 86 where applicable.
only), F 799, F 1108, or F 1537.
8.2 Metallic Bearing Surface—Articulate surfaces shall be
5.3 All polymeric components shall conform to the follow-
finished to an average roughness of 0.125 μm.
ing specification for implant materials: Specification F 648.
8.3 Polymeric Bearing Surface Finish— shall conform to
5.4 All solid ceramic components shall conform to Specifi-
manufacturer’s documented standards concerning concentric-
cation F 603 for implant materials.
ity, sphericity, and surface roughness, when applicable.
5.5 Biocompatibility—Articulating implants shall be manu- 8.4 Items conforming to this specification shall be marked
factured from the materials listed in 5.2-5.4. Before implants in accordance with Practices F 86, and F 983. Radial and carpal
can be manufactured from other materials, their biocompatibil- component marking shall include, as possible, the items below
in the following order of importance:
ity will be considered suitable only if they produce an
acceptable response after testing in accordance with Practice 8.4.1 Manufacturer,
8.4.2 Size,
F 981.
8.4.3 Catalog Number,
5.6 When required for metallic implants, fluorescent pen-
8.4.4 Lot Number, and
etrant inspection shall be performed in accordance with Prac-
8.4.5 Orientation (dorsal/palmar/radial/ulnar/left/right as
tice F 601.
appropriate).
5.7 When required for cast metallic implants, radiography
8.5 If one of the components is not radiographic opaque, it
shall be performed in accordance with Practice F 629.
shall contain a marker wire or other means of radiographic
detection. If used, it may be located at the manufacturer’s
6. Performance Requirements
discretion.
6.1 Polymeric Creep (Cold Flow)—Ultra-high molecular
9. Packaging and Package Marking
weight polyethylene in implant form must conform to the
requirements detailed in Specification F 648. When creep
9.1 The maximum range of motion values as determined by
occurs, it must not impair the function or stability of the
6.3 shall be included in the product labeling.
interface.
9.2 The dimensions shown in Figs. 1 and 2 and described in
the glossary in Appendix X1 shall be included in the product
6.2 Wear of Alternative Materials—It is important to under-
labeling.
stand the wear performance for articulating surfaces. Any new
or different material couple should not exceed the wear rates of
the following material couple when tested under physiological
conditions. The current wear couple is CoCrMo alloy (F75)
against ultra high molecular weight polyethylene. This is an
industry wide referenced wear couple and is considered by
some to be the minimum. It has been proven to provide
clinically acceptable results.
NOTE 1—In situations where the pin-on-flat test may not be considered
appropriate, other test methods may be considered.
6.3 Range of Motion of the Device Before Implantation—
The implant shall be evaluated to determine the maximum
dorsiflexion, palmar flexion, radial deviation, and ulnar devia-
tion possible before subluxation occurs or the motion is
arrested by the implant. These results shall be reported in the
product labeling.
6.4 Guidelines for In-Vitro Laboratory Testing—No ASTM
standards for testing articulating wrist implants have not been
developed. Laboratory testing that simulates the conditions of
use is desirable to compare materials and designs and to
provide an indication of clinical performance. Implant testing
shall be done in keeping with the implants intended function,
that is, implants intended to partially stabilize or stabilize a
join
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.