ASTM D3973-85(2011)
(Test Method)Standard Test Method for Low-Molecular Weight Halogenated Hydrocarbons in Water
Standard Test Method for Low-Molecular Weight Halogenated Hydrocarbons in Water
SIGNIFICANCE AND USE
The incidental conversion of organic material to trihalomethanes and other volatile organohalides during chlorination of water is a possible health hazard and is the object of much research. This test method can be used as a rapid, simple means for determining many volatile organohalides in raw and processed water.
SCOPE
1.1 This test method covers the analysis of drinking water. It is also applicable to many environmental and waste waters when adequate validation is included.
1.2 This test method covers the determination of halomethanes, haloethanes, and some related extractable organohalides amenable to gas chromatographic measurement. The applicable concentration range for trihalomethanes is from 1 to 200 μg/L. Detection limits depend on the compound, matrix, and on the characteristics of the gas chromatographic system.
1.3 For compounds not specifically included in the precision and bias section the analyst should validate the test method by collecting precision and bias data on actual samples.
1.4 Confirmation of component identities is obtained by observing retention times using gas chromatographic columns of different polarities. When concentrations are sufficiently high (>50 μg/L) confirmation with halogen specific detectors or gas chromatography/mass spectrometry (GC/MS) may be used. Confirmation of purgeable compounds at levels down to 1 μg/L can be obtained using Test Method D3871 with GC/MS detection.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in Section 8.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D3973 − 85 (Reapproved 2011)
Standard Test Method for
Low-Molecular Weight Halogenated Hydrocarbons in Water
This standard is issued under the fixed designation D3973; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This test method covers the analysis of drinking water.
2.1 ASTM Standards:
It is also applicable to many environmental and waste waters D1129 Terminology Relating to Water
when adequate validation is included.
D1193 Specification for Reagent Water
D3871 Test Method for Purgeable Organic Compounds in
1.2 This test method covers the determination of
Water Using Headspace Sampling
halomethanes, haloethanes, and some related extractable or-
E355 Practice for Gas ChromatographyTerms and Relation-
ganohalides amenable to gas chromatographic measurement.
ships
The applicable concentration range for trihalomethanes is from
1 to 200 µg/L. Detection limits depend on the compound,
3. Terminology
matrix, and on the characteristics of the gas chromatographic
system.
3.1 Definitions—For definitions of terms used in this test
method, refer to Terminology D1129 and Practice E355.
1.3 Forcompoundsnotspecificallyincludedintheprecision
and bias section the analyst should validate the test method by
4. Summary of Test Method
collecting precision and bias data on actual samples.
4.1 This test method employs liquid/liquid extraction to
1.4 Confirmation of component identities is obtained by
isolate compounds of interest and provide a five-fold concen-
observing retention times using gas chromatographic columns
3, 4, 5
tration enhancement prior to measurement. A5-mL aque-
of different polarities. When concentrations are sufficiently
ous sample is extracted once with 1 mL of solvent. D3871-µL
high (>50 µg/L) confirmation with halogen specific detectors
aliquot of the extract is analyzed in a gas chromatograph
or gas chromatography/mass spectrometry (GC/MS) may be
equipped with an electron-capture detector.
used. Confirmation of purgeable compounds at levels down to
1 µg/Lcan be obtained usingTest Method D3871 with GC/MS
4.2 Extraction efficiencies with the 1:5 solvent/sample ratio
detection.
for trihalomethanes average above 90 %. To compensate for
extraction losses, calibration standards are extracted and ana-
1.5 The values stated in SI units are to be regarded as
lyzed in an identical manner.
standard. No other units of measurement are included in this
standard.
4.3 The concentration of each component is calculated and
1.6 This standard does not purport to address all of the reported in micrograms per litre.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
bility of regulatory limitations prior to use. Specific precau-
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
tionary statements are given in Section 8. Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
Mieure, J. P., “A Rapid and Sensitive Method for Determining Volatile
This test method is under the jurisdiction of ASTM Committee D19 on Water Organohalides in Water,” Journal AWWA, Vol 69, 1977, p. 60.
andisthedirectresponsibilityofSubcommitteeD19.06onMethodsforAnalysisfor Richard, J. J., and Junk, G. A., “Liquid Extraction for Rapid Determination of
Organic Substances in Water. Halomethanes in Water,” Journal AWWA , Vol 69, 1977, p. 62.
Current edition approved May 1, 2011. Published June 2011. Originally “The Analysis of Trihalomethanes in Drinking Water by Liquid/Liquid
approved in 1980. Last previous edition approved in 2003 as D3973 – 85 (2003). Extraction,” U.S. Environmental Protection Agency, EMSL, Cincinnati, OH, Sept.
DOI: 10.1520/D3973-85R11. 9, 1977.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3973 − 85 (2011)
5. Significance and Use 8. Reagents
5.1 The incidental conversion of organic material to triha- 8.1 Purity of Reagents—Reagent grade chemicals shall be
lomethanes and other volatile organohalides during chlorina- used in all tests. Unless otherwise indicated, it is intended that
tion of water is a possible health hazard and is the object of all reagents shall conform to the specifications of the Commit-
much research.This test method can be used as a rapid, simple tee on Analytical Reagents of the American Chemical
means for determining many volatile organohalides in raw and Society, wheresuchspecificationsareavailable.Othergrades
processed water. may be used, provided it is first ascertained that the reagent is
of sufficiently high purity to permit its use without lessening
6. Interferences
the accuracy of the determination.
6.1 Volatile compounds that are extractable and responsive
8.2 Purity of Water—Unless otherwise indicated, Specifica-
to electron-capture detection may interfere with this test
tion D1193 reagent water, Type II, will be used in this test
method.
method. In addition the water is made organic-free by passing
it through a filter bed containing about 0.4 kg of activated
6.2 Impurities in the extracting solvent can be a source of
carbon (8.3), or using a commercial water purification sys-
interference. Solvent blanks should be analyzed daily and
tem.
before a new bottle of solvent is used for the first time.
Whenever interfering compounds are traced to the solvent, a
8.3 Activated Carbon.
new source of solvent should be obtained. Alternatively,
8.4 Dechlorinating Agent—Granular sodium thiosulfate or
impurities may be removed by distillation, column chromatog-
ascorbic acid.
raphy or purging with high-purity nitrogen or helium. This
8.5 Detergent, suitable for laboratory glassware.
procedure is quantitative as long as solvent interference con-
tributes less than 10 % to the component concentration in the
8.6 Isooctane, pesticide grade.
sample.
8.7 Methyl Alcohol, pesticide grade.
7. Apparatus 8.8 Sodium Chloride, granular.
7.1 Extraction Vessel, 9-mL (2-dram) vial with aluminum
8.9 Reference Standards:
foil or PTFE-lined caps. 8.9.1 Bromoform.
8.9.2 Bromodichloromethane.
7.2 Sample Containers, 40-mL screw cap vials sealed with
8.9.3 Chlorodibromomethane.
PTFE-faced silicone septa.
8.9.4 Chloroform.
7.3 Micro Syringes, 10, 100-µL.
8.9.5 Tetrachloroethylene.
7.4 Pipets, 1.0 and 5.0-mL transfer. 8.9.6 1,1,1-Trichloroethane.
7.5 Glass-Stoppered Volumetric Flasks, 10 and 100-mL. 8.10 Stock Solutions—Prepare a stock solution (2 to 10
mg/mL) for each standard as follows:
7.6 Gas Chromatograph, with electron-capture detector.
8.10.1 Warning—Because of the toxicity of trihalometh-
7.7 Columns, either of the following columns have been
anes it is necessary to prepare primary dilutions in a hood. It is
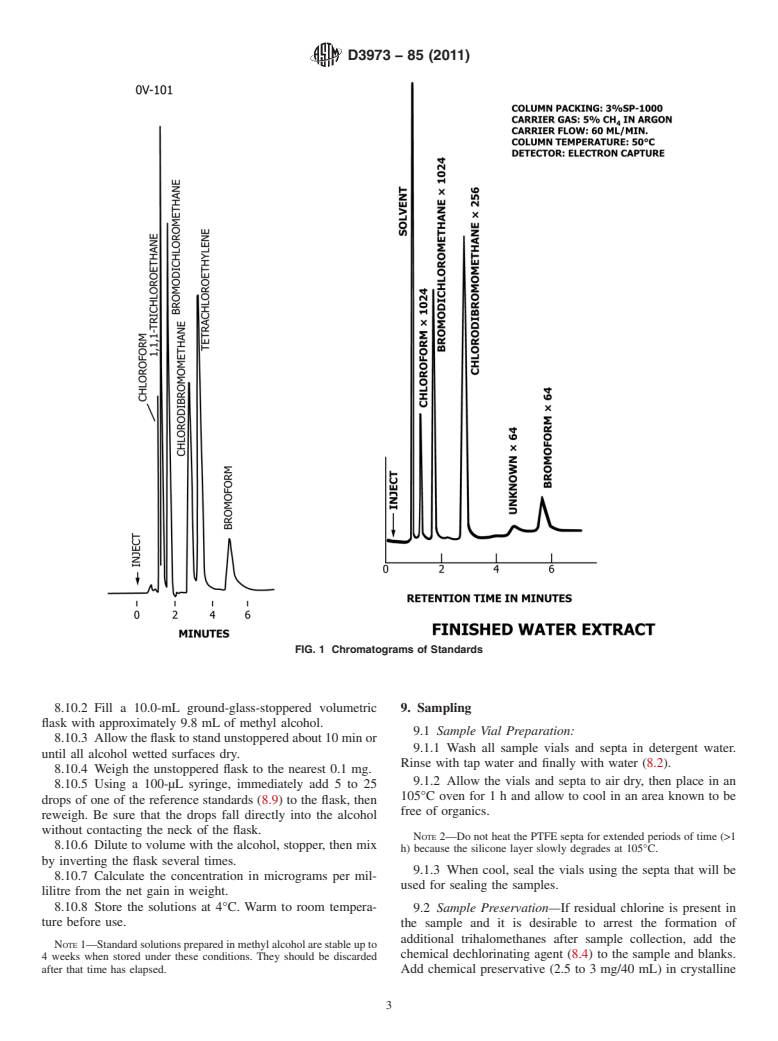
found suitable for this analysis. See Fig. 1 for chromatograms.
furtherrecommendedthataNIOSH/MESA-approvedtoxicgas
If other column conditions are used, it is up to the analyst to
respirator be used when the analyst handles high concentra-
demonstrate the precision and bias statements are met by
tions of such materials.
collecting precision, bias, and recovery data.
7.7.1 Nonpolar, 3-mm inside diameter by 2-m long boro-
Reagent Chemicals, American Chemical Society Specifications , American
silicate glass packed with 100/120 mesh support coated with
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
a methyl silicone liquid phase and operated at 60°C with 45
listed by the American Chemical Society, see Analar Standards for Laboratory
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
mL/min carrier flow.
and National Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville,
7.7.2 Polar, 3-mm inside diameter by 2-m long borosilicate
MD.
glass packed with 100/120 mesh support coated with a polar
Super Q water system, available from Millipore Corp. Ashby Rd., Bedford,
MA 01730, has been found suitable; other sources that are equivalent may be
liquid phase such as polyethylene glycol and operated at 50°C
substituted.
with 60 mL/min carrier flow.
Filtrasorb 200, available from Calgon Corp., Box 1346, Pittsburgh, PA15230,
has been found suitable; other sources that are equivalent may be substituted.
Pesticide grade, available from Burdick & Jackson Labs, Inc., 1953 S. Harvey
13075 vials and 12722 septa, available from Pierce Chemical Co., P.O. Box St., Muskegon, MI 49442, or Spectro Grade, available from Phillips Chemical Co.,
117,Rockford,IL61105,havebeenfoundsuitable;othersourcesthatareequivalent Specialty Chemicals, Drawer “O”, Borger, TX 79007, have been found suitable;
may be substituted. other sources that are equivalent may be substituted.
7 14
Gas-Chrom Q, available fromApplied Science Laboratory, Inc., P.O. Box 440, Pesticide grade, available from Burdick & Jackson Labs, Inc., has been found
State College, PA16801, has been found suitable; other sources that are equivalent suitable; other sources which are equivalent may be substituted.
may be substituted. Bromodichloromethane,availablefromAldrichChemicalCo.,Inc.,940W.St.
OV-101, available from Ohio Valley Specialty Chemical, Inc., Route 6, Paul Ave., Milwaukee, WI 53233, has been found suitable; other sources that are
Marietta, OH 45750, has been found suitable; other sources that are equivalent may equivalent may be substituted.
be substituted. Chlorodibromomethane, available from Chemical Service Inc., 1887 Lincoln
SP-1000,availablefromSupelco,Inc.,SupelcoPark,Bellafonte,PA16823,has Ave., West Chester, PA, has been found suitable; other sources that are equivalent
been found suitable; other sources that are equivalent may be substituted. may be substituted.
D3973 − 85 (2011)
FIG. 1 Chromatograms of Standards
8.10.2 Fill a 10.0-mL ground
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.