ASTM F2791-24
(Guide)Standard Guide for Assessment of Surface Texture of Non-Porous Biomaterials in Two Dimensions
Standard Guide for Assessment of Surface Texture of Non-Porous Biomaterials in Two Dimensions
SIGNIFICANCE AND USE
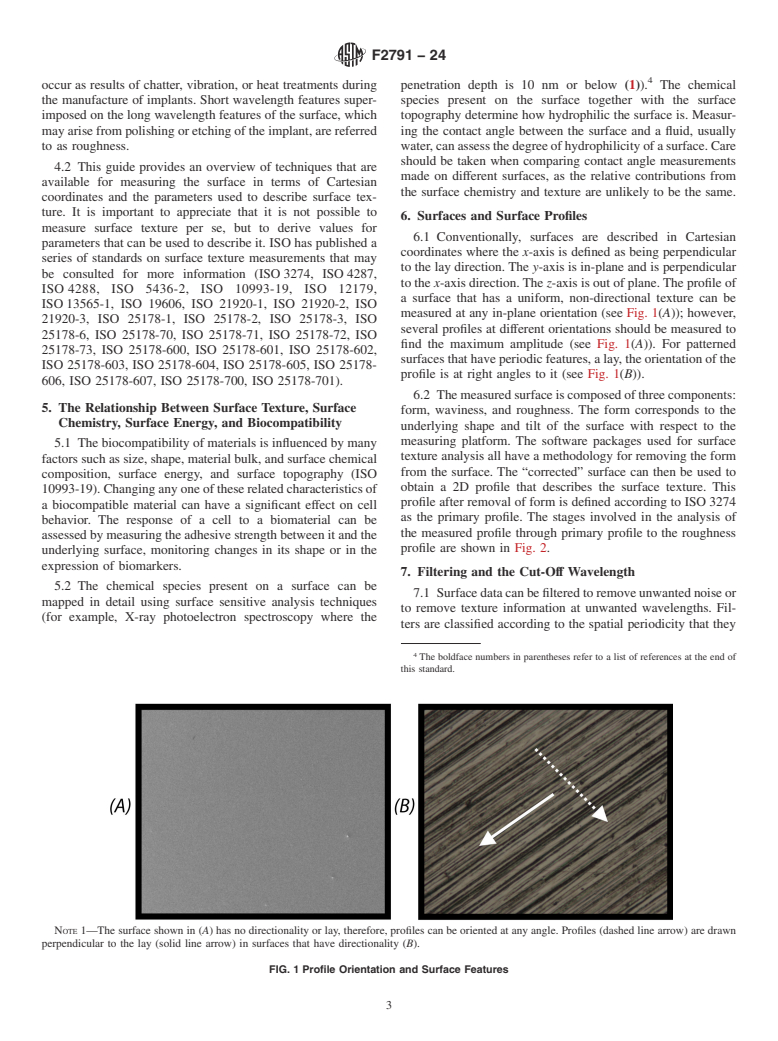
4.1 The term “surface texture” is used to describe the local deviations of a surface from an ideal shape. Surface texture usually consists of long wavelength repetitive features that occur as results of chatter, vibration, or heat treatments during the manufacture of implants. Short wavelength features superimposed on the long wavelength features of the surface, which may arise from polishing or etching of the implant, are referred to as roughness.
4.2 This guide provides an overview of techniques that are available for measuring the surface in terms of Cartesian coordinates and the parameters used to describe surface texture. It is important to appreciate that it is not possible to measure surface texture per se, but to derive values for parameters that can be used to describe it. ISO has published a series of standards on surface texture measurements that may be consulted for more information (ISO 3274, ISO 4287, ISO 4288, ISO 5436-2, ISO 10993-19, ISO 12179, ISO 13565-1, ISO 19606, ISO 21920-1, ISO 21920-2, ISO 21920-3, ISO 25178-1, ISO 25178-2, ISO 25178-3, ISO 25178-6, ISO 25178-70, ISO 25178-71, ISO 25178-72, ISO 25178-73, ISO 25178-600, ISO 25178-601, ISO 25178-602, ISO 25178-603, ISO 25178-604, ISO 25178-605, ISO 25178-606, ISO 25178-607, ISO 25178-700, ISO 25178-701).
SCOPE
1.1 This guide describes some of the more common methods that are available for measuring the topographical features of a surface and provides an overview of the parameters that are used to quantify them. Being able to reliably derive a set of parameters that describe the texture of biomaterial surfaces is a key aspect in the manufacture of safe and effective implantable medical devices that have the potential to trigger an adverse biological reaction in situ.
1.2 This guide is not intended to apply to porous structures with average pore dimensions in excess of approximately 50 nm (0.05 μm).
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2791 − 24
Standard Guide for
Assessment of Surface Texture of Non-Porous Biomaterials
1
in Two Dimensions
This standard is issued under the fixed designation F2791; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope F2312 Terminology Relating to Tissue Engineered Medical
Products
1.1 This guide describes some of the more common meth-
F2664 Guide for Assessing the Attachment of Cells to
ods that are available for measuring the topographical features
Biomaterial Surfaces by Physical Methods
of a surface and provides an overview of the parameters that
3
2.2 Other Standards:
are used to quantify them. Being able to reliably derive a set of
ISO 3274 Geometrical Product Specifications (GPS)—
parameters that describe the texture of biomaterial surfaces is
Surface Texture: Profile Method—Nominal Characteris-
a key aspect in the manufacture of safe and effective implant-
tics of Contact (Stylus) Instruments
able medical devices that have the potential to trigger an
ISO 4287 Geometrical Product Specifications (GPS)—
adverse biological reaction in situ.
Surface Texture: Profile Method—Terms, Definitions and
1.2 This guide is not intended to apply to porous structures
Surface Texture Parameters
with average pore dimensions in excess of approximately
ISO 4288 Geometrical Product Specifications (GPS)—
50 nm (0.05 μm).
Surface Texture: Profile Method—Rules and Procedures
1.3 The values stated in SI units are to be regarded as
for the Assessment of Surface Texture
standard. No other units of measurement are included in this
ISO 5436-2 Geometrical Product Specifications (GPS)—
standard.
Surface Texture: Profile Method; Measurement
Standards—Part 2: Software Measurement Standards
1.4 This standard does not purport to address all of the
ISO 10993-19 Biological Evaluation of Medical Devices—
safety concerns, if any, associated with its use. It is the
Part 19: Physico-chemical, Morphological and Topo-
responsibility of the user of this standard to establish appro-
graphical Characterization of Materials
priate safety, health, and environmental practices and deter-
ISO 12179 Geometrical Product Specifications (GPS)—
mine the applicability of regulatory limitations prior to use.
Surface Texture: Profile Method—Calibration of Contact
1.5 This international standard was developed in accor-
(Stylus) Instruments
dance with internationally recognized principles on standard-
ISO 13565-1 Geometrical Product Specifications (GPS)—
ization established in the Decision on Principles for the
Surface Texture: Profile Method—Surfaces Having Strati-
Development of International Standards, Guides and Recom-
fied Functional Properties; Filtering and General Measure-
mendations issued by the World Trade Organization Technical
ment Conditions
Barriers to Trade (TBT) Committee.
ISO 19606 Fine Ceramics (Advanced Ceramics, Advanced
Technical Ceramics)—Test Method for Surface Rough-
2. Referenced Documents
ness of Fine Ceramic Films by Atomic Force Microscopy
2
2.1 ASTM Standards:
ISO 21920-1 Geometrical Product Specifications (GPS)—
C813 Test Method for Hydrophobic Contamination on Glass
Surface Texture: Profile—Part 1: Indication of Surface
by Contact Angle Measurement
Texture
ISO 21920-2 Geometrical Product Specifications (GPS)—
Surface Texture: Profile—Part 2: Terms, Definitions and
1
This guide is under the jurisdiction of ASTM Committee F04 on Medical and
Surface Texture Parameters
Surgical Materials and Devices and is the direct responsibility of Subcommittee
ISO 21920-3 Geometrical Product Specifications (GPS)—
F04.42 on Biomaterials and Biomolecules for TEMPs.
Current edition approved March 15, 2024. Published March 2024. Originally Surface Texture: Profile—Part 3: Specification Operators
approved in 2009. Last previous edition approved in 2015 as F2791 – 15. DOI:
ISO 25178-1 Geometrical Product Specifications (GPS)—
10.1520/F2791-24.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2791 −
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2791 − 15 F2791 − 24
Standard Guide for
Assessment of Surface Texture of Non-Porous Biomaterials
1
in Two Dimensions
This standard is issued under the fixed designation F2791; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide describes some of the more common methods that are available for measuring the topographical features of a
surface and provides an overview of the parameters that are used to quantify them. Being able to reliably derive a set of parameters
that describe the texture of biomaterial surfaces is a key aspect in the manufacture of safe and effective implantable medical devices
that have the potential to trigger an adverse biological reaction in situ.
1.2 This guide is not intended to apply to porous structures with average pore dimensions in excess of approximately 50 nm 50 nm
(0.05 μm).
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of
regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
C813 Test Method for Hydrophobic Contamination on Glass by Contact Angle Measurement
F2312 Terminology Relating to Tissue Engineered Medical Products
F2450 Guide for Assessing Microstructure of Polymeric Scaffolds for Use in Tissue-Engineered Medical Products
F2664 Guide for Assessing the Attachment of Cells to Biomaterial Surfaces by Physical Methods
3
2.2 Other Standards:
ISO 3274 Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Nominal Characteristics of Contact
(Stylus) Instruments
ISO 4287 Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Terms, Definitions and Surface
Texture Parameters
1
This guide is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee F04.42
on Biomaterials and Biomolecules for TEMPs.
Current edition approved May 1, 2015March 15, 2024. Published June 2015March 2024. Originally approved in 2009. Last previous edition approved in 20142015 as
F2791F2791 – 15.– 14. DOI: 10.1520/F2791-15. DOI: 10.1520/F2791-24.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
Available from American National Standards Institute (ANSI), 25 W. 43rd St., 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2791 − 24
ISO 4288 Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Rules and Procedures for the
Assessment of Surface Texture
ISO 5436-2 Geometrical Product Specifications (GPS)—Surface Texture: Profile Method; Measurement Standards—Part 2:
Software Measurement Standards
ISO 10993-19 Biological Evaluation of Medical Devices—Part 19: Physico-chemical, Morphological and Topographical
Characterization of Materials
ISO 12179 Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Calibration of Contact (Stylus)
Instruments
ISO 13565–1ISO 13565-1 Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Surfaces Having
Stratified Functional Properties; Filtering and General Measurement Conditions
ISO 19606 Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Surface Roughness of Fine
Ceramic Films by Atomic Force Microscopy
ISO 21920-1 Geometrical Product Specifications (GPS)—Surface Texture: Profile—Part 1: Indication of Surface Texture
ISO 21
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.