ASTM D2780-92(2007)
(Test Method)Standard Test Method for Solubility of Fixed Gases in Liquids (Withdrawn 2010)
Standard Test Method for Solubility of Fixed Gases in Liquids (Withdrawn 2010)
SIGNIFICANCE AND USE
The solubility of fixed gases in liquids is an important engineering parameter in the design of hydraulic systems. It is a measure of the amount of gas which can be released from solution when a system undergoes changes in pressure and temperature. Theoretical considerations permit approximate values of gas solubility to be computed with reasonable accuracy. In this test method, dissolved gases are separated physically from a liquid and measured volumetrically. The test method permits subsequent analysis of separated gases by any appropriate method.
SCOPE
1.1 This test method covers the determination of the solubility of fixed gases in liquids. It is suitable for gases and liquids that do not react with each other and are compatible with borosilicate glass, mercury, stainless steel, PTFE (polytetrafluoroethylene), and FPM (vinylidene fluoride-hexafluoro propylene copolymer) under the conditions of the test. This test method also covers the determination of the concentration of fixed gases in solutions which are not saturated with the gas.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements see 6.1, 6.2, 8.3, 8.4.2, and 9.3.
1.2 The values stated in SI units are to be regarded as the standard. The values in parentheses are for information only.
WITHDRAWN RATIONALE
This test method covers the determination of the solubility of fixed gases in liquids. It is suitable for gases and liquids that do not react with each other and are compatible with borosilicate glass, mercury, stainless steel, PTFE (polytetrafluoroethylene), and FPM (vinylidene fluoride-hexafluoro propylene copolymer) under the conditions of the test. This test method also covers the determination of the concentration of fixed gases in solutions which are not saturated with the gas.
Formerly under the jurisdiction of Committee D02 on Petroleum Products and Lubricants, this test method was withdrawn in October 2010 due to its use of large amounts of mercury.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D2780–92(Reapproved 2007)
Standard Test Method for
Solubility of Fixed Gases in Liquids
This standard is issued under the fixed designation D2780; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope presented for analysis.Aportion of the solution of gas in liquid
is transferred to a gas extraction apparatus in which the fixed
1.1 This test method covers the determination of the solu-
gas is quantitatively removed from the liquid. The separated
bility of fixed gases in liquids. It is suitable for gases and
gas is transferred to a gas buret in which its volume is
liquids that do not react with each other and are compatible
determined.
with borosilicate glass, mercury, stainless steel, PTFE (poly-
tetrafluoroethylene), and FPM (vinylidene fluoride-hexafluoro
4. Significance and Use
propylenecopolymer)undertheconditionsofthetest.Thistest
4.1 The solubility of fixed gases in liquids is an important
method also covers the determination of the concentration of
engineering parameter in the design of hydraulic systems. It is
fixed gases in solutions which are not saturated with the gas.
a measure of the amount of gas which can be released from
1.2 This standard does not purport to address all of the
solution when a system undergoes changes in pressure and
safety concerns, if any, associated with its use. It is the
temperature. Theoretical considerations permit approximate
responsibility of the user of this standard to establish appro-
values of gas solubility to be computed with reasonable
priate safety and health practices and determine the applica-
accuracy. In this test method, dissolved gases are separated
bility of regulatory limitations prior to use. For specific hazard
physically from a liquid and measured volumetrically. The test
statements see 6.1, 6.2, 8.3, 8.4.2, and 9.3.
method permits subsequent analysis of separated gases by any
1.3 The values stated in SI units are to be regarded as the
appropriate method.
standard. The values in parentheses are for information only.
5. Apparatus
2. Referenced Documents
2 5.1 Ambient Pressure Saturator, suitable for the saturation
2.1 ASTM Standards:
of liquids with fixed gases at various temperatures at ambient
D831 Test Method for Gas Content of Cable and Capacitor
pressure is shown in Fig. 1. The system comprises four parts:
Oils
5.1.1 Gas Supply and Pressure Regulator,
D2883 Test Method for Reaction Threshold Temperature of
5.1.2 Gas Dispersion Element,
Liquid and Solid Materials
5.1.3 Heating Mantle, to fit 1000-mL separatory funnel
D4057 Practice for Manual Sampling of Petroleum and
(Fig. 1), and
Petroleum Products
5.1.4 Temperature Measurement and Control Devices.
E260 Practice for Packed Column Gas Chromatography
NOTE 1—In the event that it is desired to saturate a liquid with a toxic
3. Summary of Test Method
or flammable gas, the use of this system is not recommended, unless
suitable means are provided for the collection and disposal of the escaping
3.1 Aspecimenofthetestliquidissaturatedwithafixedgas
gas.
under specified conditions of temperature and pressure. The
saturation step may be eliminated if it is desired to determine
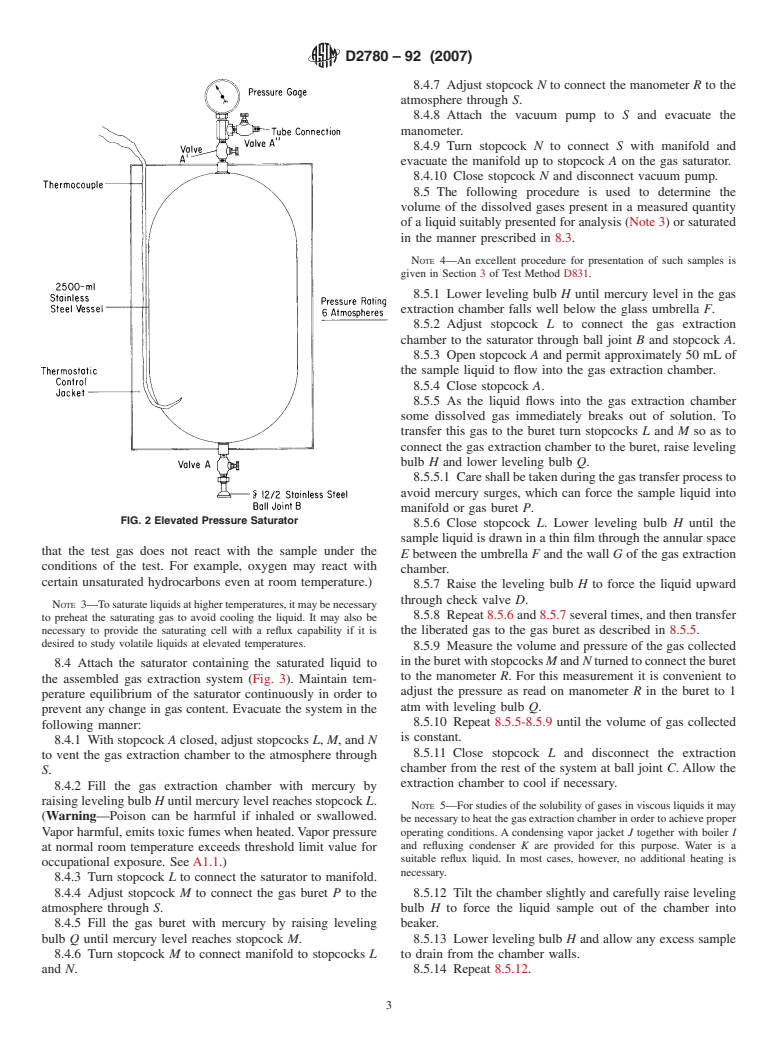
5.2 Elevated Pressure Saturator, used to saturate liquids
the concentration of fixed gas in a liquid sample suitably
with gases at pressures other than ambient. A suitable vessel,
usable at pressures up to 608 kPa (6 atm), is illustrated in Fig.
2. The vessel consists of a 2.5 L stainless steel bomb with a
This test method is under the jurisdiction of ASTM Committee D02 on
thermostatic control jacket.Avalve at one end is connected to
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
D02.11 on Engineering Sciences of High Performance Fluids and Solids. a pressure gage and gas supply. A valve at the other end is
Current edition approved May 1, 2007. Published June 2007. Originally
provided with a fitting that connects directly to the gas
´1
approved in 1969. Last previous edition approved in 2002 as D2780 – 92 (2002) .
extraction apparatus.
DOI: 10.1520/D2780-92R07.
5.2.1 Thermostatic Control, for jacket of saturator.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
5.2.2 Shaker, reciprocating, horizontal.
Standards volume information, refer to the standard’s Document Summary page on
5.2.3 Vacuum Pump, rotary.
the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2780–92 (2007)
FIG. 1 Ambient Pressure Saturator
5.2.4 TransferLine, with two male socket joint 12/2 fittings. pressure at normal room temperature exceeds threshold limit
5.3 GasExtractionSystem, as shown schematically in Fig. value for occupational exposure. See A1.1.)
3. A detailed drawing of the extraction chamber is shown in 6.2 Compressed Gases, as required for saturating liquids to
Fig. 4. The apparatus provides for the separation of dissolved be studied. (Warning—Compressed gas under high pressure.
gases from a liquid by repeatedly forcing the liquid containing Gas reduces oxygen available for breathing. See A1.2.)
gas to pass through a narrow annular passage under reduced
7. Sampling
pressure. Gas removed in this manner is stored and measured
in a gas buret. Provision is made for heating the extraction 7.1 To obtain specimens for total gas solubility measure-
chamber by means of a condensing vapor bath. The gas buret
ments, collect samples in accordance with Practice D4057. For
is jacketed. Cooling water may be circulated through the jacket the determination of the concentration of fixed gases in
if it is necessary to reduce the temperature of the contents of solutions which are not saturated with the gas, take samples in
the buret.Amanometer is attached to the manifold connecting
accordance with the procedure described in Section 3 of Test
the saturation system, gas extractor, and gas buret. Grease-free Method D831.
stopcocks and ball joints are used throughout the system (Note
8. Procedure A
2). All tubing and connections are 1 mm inside diameter.
8.1 ProcedureAcoversthedeterminationofthesolubilityof
NOTE 2—PTFE stopcocks are satisfactory for most purposes. However,
fixed gases in liquids at ambient pressure.
for greatest precision construct the apparatus with stopcocks and joints
8.2 Add to the ambient pressure saturator (Fig. 1) a suffi-
which are fitted with O-ring seals.
cient amount of the liquid to cover the gas dispersion element
6. Reagents and Materials
with at least 50 to 80 mm of liquid. Bring the cell to
6.1 Mercury, triple-distilled, instrument-grade, sufficient temperature equilibrium at whatever temperature is desired for
amount to fill extraction apparatus, gas buret, and leveling the determination.
bulbs. (Warning—Poison can be harmful if inhaled or swal- 8.3 Saturatetheliquidwiththetestgas(Warning—See6.2)
lowed. Vapor harmful, emits toxic fumes when heated. Vapor by bubbling the gas through the liquid.Adjust the gas flow rate
so that the gas stream causes thorough but not violent agitation
of the liquid. If saturation is to be carried out at an elevated
temperature, it may be necessary to reestablish temperature
The gas extraction system is similar to that described by J. H. D. Hooper, API
Proceedings, 1948. equilibrium after the start of gas flow. (Warning—Be certain
D2780–92 (2007)
8.4.7 Adjust stopcock N to connect the manometer R to the
atmosphere through S.
8.4.8 Attach the vacuum pump to S and evacuate the
manometer.
8.4.9 Turn stopcock N to connect S with manifold and
evacuate the manifold up to stopcock A on the gas saturator.
8.4.10 Close stopcock N and disconnect vacuum pump.
8.5 The following procedure is used to determine the
volume of the dissolved gases present in a measured quantity
of a liquid suitably presented for analysis (Note 3) or saturated
in the manner prescribed in 8.3.
NOTE 4—An excellent procedure for presentation of such samples is
given in Section 3 of Test Method D831.
8.5.1 Lower leveling bulb H until mercury level in the gas
extraction chamber falls well below the glass umbrella F.
8.5.2 Adjust stopcock L to connect the gas extraction
chamber to the saturator through ball joint B and stopcock A.
8.5.3 Open stopcock A and permit approximately 50 mL of
the sample liquid to flow into the gas extraction chamber.
8.5.4 Close stopcock A.
8.5.5 As the liquid flows into the gas extraction chamber
some dissolved gas immediately breaks out of solution. To
transfer this gas to the buret turn stopcocks L and M so as to
connect the gas extraction chamber to the buret, raise leveling
bulb H and lower leveling bulb Q.
8.5.5.1 Care shall be taken during the gas transfer process to
avoid mercury surges, which can force the sample liquid into
manifold or gas buret P.
FIG. 2 Elevated Pressure Saturator
8.5.6 Close stopcock L. Lower leveling bulb H until the
sample liquid is drawn in a thin film through the annular space
that the test gas does not react with the sample under the
E between the umbrella F and the wall G of the gas extraction
conditions of the test. For example, oxygen may react with
chamber.
certain unsaturated hydrocarbons even at room temperature.)
8.5.7 Raise the leveling bulb H to force the liquid upward
through check valve D.
NOTE 3—Tosaturateliquidsathighertemperatures,itmaybenecessary
8.5.8 Repeat 8.5.6 and 8.5.7 several times, and then transfer
to preheat the saturating gas to avoid cooling the liquid. It may also be
necessary to provide the saturating cell with a reflux capability if it is the liberated gas to the gas buret as described in 8.5.5.
desired to study volatile liquids at elevated temperatures.
8.5.9 Measure the volume and pressure of the gas collected
intheburetwithstopcocksMandNturnedtoconnecttheburet
8.4 Attach the saturator containing the saturated liquid to
to the manometer R. For this measurement it is convenient to
the assembled gas extraction system (Fig. 3). Maintain tem-
adjust the pressure as read on manometer R in the buret to 1
perature equilibrium of the saturator continuously in order to
atm with leveling bulb Q.
prevent any change in gas content. Evacuate the system in the
8.5.10 Repeat 8.5.5-8.5.9 until the volume of gas collected
following manner:
is constant.
8.4.1 With stopcock A closed, adjust stopcocks
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.