ASTM G71-81(2014)
(Guide)Standard Guide for Conducting and Evaluating Galvanic Corrosion Tests in Electrolytes
Standard Guide for Conducting and Evaluating Galvanic Corrosion Tests in Electrolytes
SIGNIFICANCE AND USE
3.1 Use of this guide is intended to provide information on the galvanic corrosion of metals in electrical contact in an electrolyte that does not have a flow velocity sufficient to cause erosion-corrosion or cavitation.
3.2 This standard is presented as a guide for conducting galvanic corrosion tests in liquid electrolyte solutions, both in the laboratory and in service environments. Adherence to this guide will aid in avoiding some of the inherent difficulties in such testing.
SCOPE
1.1 This guide covers conducting and evaluating galvanic corrosion tests to characterize the behavior of two dissimilar metals in electrical contact in an electrolyte under low-flow conditions. It can be adapted to wrought or cast metals and alloys.
1.2 This guide covers the selection of materials, specimen preparation, test environment, method of exposure, and method for evaluating the results to characterize the behavior of galvanic couples in an electrolyte. Note 1—Additional information on galvanic corrosion testing and examples of the conduct and evaluation of galvanic corrosion tests in electrolytes are given in Refs (1)2 through (2).
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G71 − 81 (Reapproved 2014)
Standard Guide for
Conducting and Evaluating Galvanic Corrosion Tests in
Electrolytes
ThisstandardisissuedunderthefixeddesignationG71;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope G16 Guide for Applying Statistics to Analysis of Corrosion
Data
1.1 This guide covers conducting and evaluating galvanic
G31 Guide for Laboratory Immersion Corrosion Testing of
corrosion tests to characterize the behavior of two dissimilar
Metals
metals in electrical contact in an electrolyte under low-flow
G46 Guide for Examination and Evaluation of Pitting Cor-
conditions. It can be adapted to wrought or cast metals and
rosion
alloys.
1.2 This guide covers the selection of materials, specimen
3. Significance and Use
preparation,testenvironment,methodofexposure,andmethod
3.1 Use of this guide is intended to provide information on
for evaluating the results to characterize the behavior of
the galvanic corrosion of metals in electrical contact in an
galvanic couples in an electrolyte.
electrolytethatdoesnothaveaflowvelocitysufficienttocause
NOTE 1—Additional information on galvanic corrosion testing and erosion-corrosion or cavitation.
examples of the conduct and evaluation of galvanic corrosion tests in
2 3.2 This standard is presented as a guide for conducting
electrolytes are given in Refs (1) through (2).
galvanic corrosion tests in liquid electrolyte solutions, both in
1.3 The values stated in SI units are to be regarded as
the laboratory and in service environments. Adherence to this
standard. No other units of measurement are included in this
guide will aid in avoiding some of the inherent difficulties in
standard.
such testing.
1.4 This standard does not purport to address all of the
4. Test Specimens
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
4.1 Material—Test specimens should be manufactured from
priate safety and health practices and determine the applica-
thesamematerialasthoseusedintheserviceapplicationbeing
bility of regulatory limitations prior to use.
modeled. Minor compositional or processing differences be-
tween materials or between different heats can greatly affect
2. Referenced Documents
the results in some cases.
2.1 ASTM Standards:
4.2 Size and Shape:
G1 Practice for Preparing, Cleaning, and Evaluating Corro-
4.2.1 Thesizeandshapeofthetestspecimensaredependent
sion Test Specimens
on restrictions imposed by the test location. When determining
G3 Practice for Conventions Applicable to Electrochemical
material behavior in the laboratory, it is advisable to use the
Measurements in Corrosion Testing
largest specimens permissible within the constraints of the test
G4 Guide for Conducting Corrosion Tests in Field Applica-
equipment.Ingeneral,theratioofsurfaceareatometalvolume
tions
should be large in order to favor maximum corrosion loss per
weight. Sufficient thickness should be employed, however, to
minimize the possibility of perforation of the specimens during
This guide is under the jurisdiction ofASTM Committee G01 on Corrosion of
the test exposure. When modeling large components, the size
Metals and is the direct responsibility of Subcommittee G01.11 on Electrochemical
Measurements in Corrosion Testing.
of the specimens should be as large as practical. When
Current edition approved May 1, 2014. Published May 2014. Originally
modeling smaller components, specimen size should be as
approved in 1981. Last previous edition approved in 2009 as G71–81(2009). DOI:
close as possible to that of the application being modeled.
10.1520/G0071-81R14.
Surface area ratio in the test should be identical to the
The boldface numbers in parentheses refer to a list of references at the end of
this standard.
application being modeled. This ratio is defined as the surface
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
area of one member of the couple divided by the surface area
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
of the other member of the couple. Only the area in contact
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. with the electrolyte (wetted area) is used in this calculation. In
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G71 − 81 (2014)
low-resistivity electrolytes, maintaining proximity between the volume of test solution may be varied to closely approximate
materials being coupled may be more important than maintain- the service application.
ing the exact area ratio. Also, with some couples, such as
5.1.2 Galvanic corrosion tests conducted for an extensive
copper coupled to aluminum, there may be effects of corrosion
period of time may exhaust important constituents of the
products washing from one electrode to another which may
original solution. Some accumulated corrosion products may
have to be considered in determining specimen placement.
act as corrosion accelerators or inhibitors.These variables may
4.2.2 Laboratory tests are normally performed on rectangu-
greatly change the end results, and replenishment of the
lar plates or on cylinders.When modeling service applications,
solution should be chosen to be representative of the service
the shapes of the couple members should approximate the
application. A test system using continuously replenished test
shapes in the application. Frequently complex shapes are
electrolytes is often the only solution to this problem.
simplified for testing purposes. The shape of the specimen is
5.1.3 Periodic measurements of the test environment should
more important in electrolytes of low conductivity, where
bemadewhenthetestdurationinafixedvolumesolutionisfor
voltage drop in the electrolyte is significant. In highly conduc-
periods of several days or longer. These observations may
tive electrolytes, the shapes of the couple members may
includetemperature,pH,O ,H S,CO ,NH ,conductivity,and
2 2 2 3
therefore deviate somewhat from the shapes in the application.
pertinent metal ion content.
4.3 Specimen Preparation:
5.2 Field Tests—Field testing should be performed in an
4.3.1 The edges of the test specimens should be prepared so
environment similar to the service environment. Periodic
as to eliminate all sheared or cold-worked metal except that
measurements of those environmental variables which could
cold-working introduced by stamping for identification. Shear-
varywithtime,suchastemperature,dissolvedO ,andsoforth,
ing will, in some cases, cause considerable attack. Therefore,
should be made.
specimens having sheared edges should not be used.The edges
should be finished by machining or polishing. The slight
6. Procedure
amount of cold working resulting from machining will not
6.1 Laboratory Versus Field Testing:
introduce any serious error.
6.1.1 Galvanic corrosion tests are conducted in the labora-
4.3.2 Specimens should be cleaned in accordance with
tory for several purposes: (1) inexpensive screening to reduce
Practice G1, or else the specimen surface condition should be
expensive field testing, (2) study of the effects of environmen-
similar to the application being modeled. The metallurgical
tal variables, and (3) study of the corrosion accelerating or
condition of the specimens should be similar to the application
protective effects of various anode/cathode surface area ratios.
being modeled. In all cases surface contamination, such as dirt,
6.1.2 The materials proven in the laboratory to be the most
grease, oil, and thick oxides, should be removed prior to
promising should also be tested in the field, since it is
weighing and exposure to the test environment.
frequently impossible to duplicate the actual service environ-
4.3.3 The specimen identification system must be one that
ment in the laboratory.
will endure throughout the test period. Edge notches, drilled
holes, stamped numbers, and tags are some of the methods
6.2 Test Procedure:
used for identification. The identification system must not
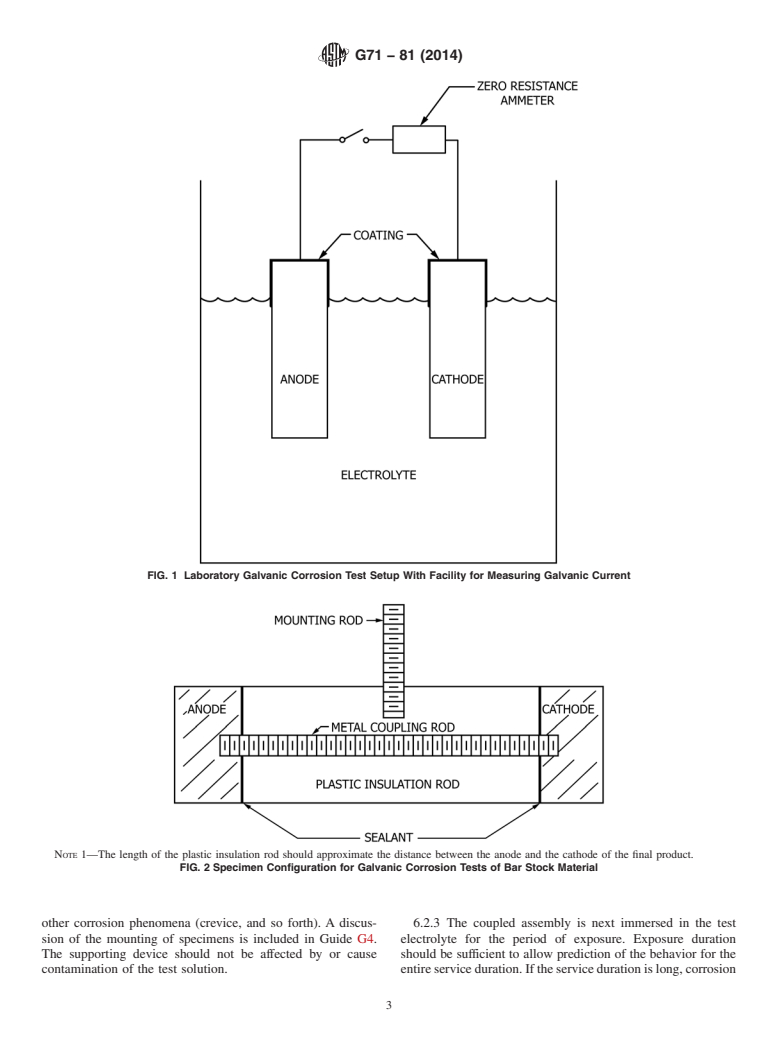
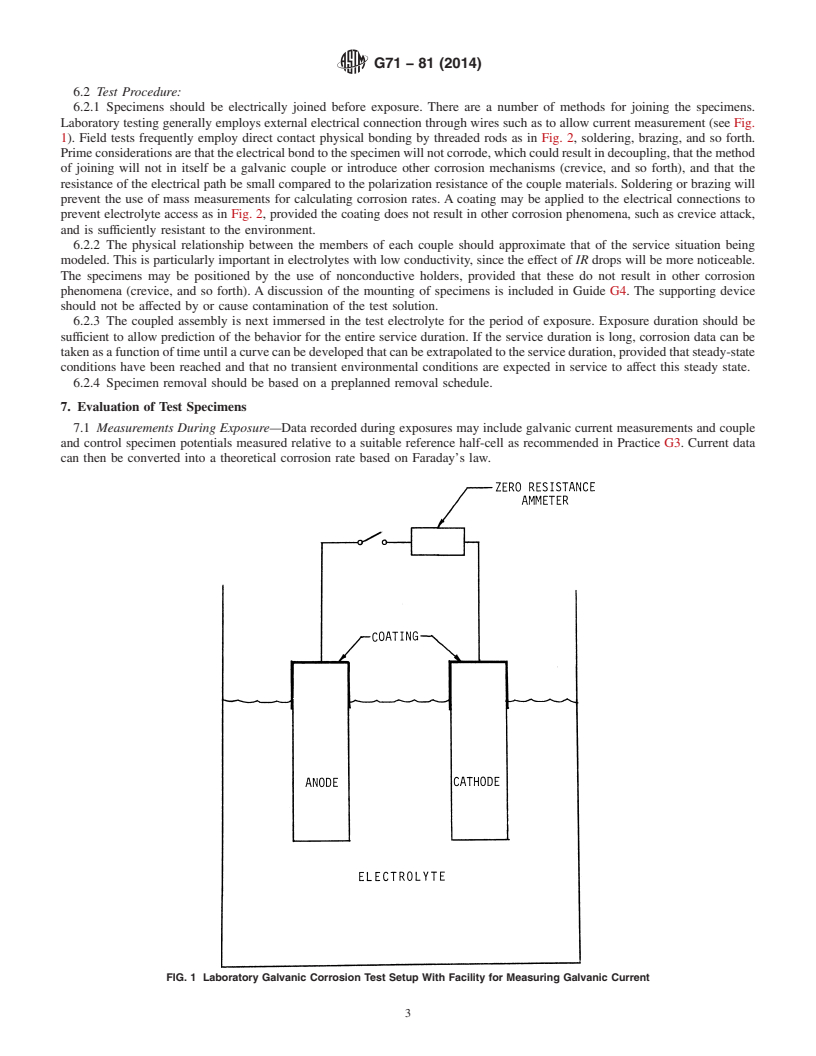
6.2.1 Specimens should be electrically joined before expo-
induce corrosion attack in any way.
sure.Thereareanumberofmethodsforjoiningthespecimens.
4.4 Number of Specimens:
Laboratory testing generally employs external electrical con-
4.4.1 The number of galvanic couples to be tested will be
nection through wires such as to allow current measurement
determined by whether or not one or more periodic specimen
(see Fig. 1). Field tests frequently employ direct contact
removals are scheduled during the course of the test. As a
physical bonding by threaded rods as in Fig. 2, soldering,
minimum, duplicate and preferably triplicate specimens should
brazing, and so forth. Prime considerations are that the
be tested for any given test period to determine the variability
electrical bond to the specimen will not corrode, which could
in the galvanic corrosion behavior. The effect of the number of
resultindecoupling,thatthemethodofjoiningwillnotinitself
replications on the application of the results is set f
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: G71 − 81 (Reapproved 2009) G71 − 81 (Reapproved 2014)
Standard Guide for
Conducting and Evaluating Galvanic Corrosion Tests in
Electrolytes
This standard is issued under the fixed designation G71; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide covers conducting and evaluating galvanic corrosion tests to characterize the behavior of two dissimilar metals
in electrical contact in an electrolyte under low-flow conditions. It can be adapted to wrought or cast metals and alloys.
1.2 This guide covers the selection of materials, specimen preparation, test environment, method of exposure, and method for
evaluating the results to characterize the behavior of galvanic couples in an electrolyte.
NOTE 1—Additional information on galvanic corrosion testing and examples of the conduct and evaluation of galvanic corrosion tests in electrolytes
are given in Refs (1) through (2).
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
G1 Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens
G3 Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing
G4 Guide for Conducting Corrosion Tests in Field Applications
G16 Guide for Applying Statistics to Analysis of Corrosion Data
G31 Guide for Laboratory Immersion Corrosion Testing of Metals
G46 Guide for Examination and Evaluation of Pitting Corrosion
3. Significance and Use
3.1 Use of this guide is intended to provide information on the galvanic corrosion of metals in electrical contact in an electrolyte
that does not have a flow velocity sufficient to cause erosion-corrosion or cavitation.
3.2 This standard is presented as a guide for conducting galvanic corrosion tests in liquid electrolyte solutions, both in the
laboratory and in service environments. Adherence to this guide will aid in avoiding some of the inherent difficulties in such testing.
4. Test Specimens
4.1 Material—Test specimens should be manufactured from the same material as those used in the service application being
modeled. Minor compositional or processing differences between materials or between different heats can greatly affect the results
in some cases.
4.2 Size and Shape:
4.2.1 The size and shape of the test specimens are dependent on restrictions imposed by the test location. When determining
material behavior in the laboratory, it is advisable to use the largest specimens permissible within the constraints of the test
This guide is under the jurisdiction of ASTM Committee G01 on Corrosion of Metals and is the direct responsibility of Subcommittee G01.11 on Electrochemical
Measurements in Corrosion Testing.
Current edition approved May 1, 2009May 1, 2014. Published May 2009May 2014. Originally approved in 1981. Last previous edition approved in 20032009 as
G71–81(2003).G71–81(2009). DOI: 10.1520/G0071-81R09.10.1520/G0071-81R14.
The boldface numbers in parentheses refer to a list of references at the end of this standard.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G71 − 81 (2014)
equipment. In general, the ratio of surface area to metal volume should be large in order to favor maximum corrosion loss per
weight. Sufficient thickness should be employed, however, to minimize the possibility of perforation of the specimens during the
test exposure. When modeling large components, the size of the specimens should be as large as practical. When modeling smaller
components, specimen size should be as close as possible to that of the application being modeled. Surface area ratio in the test
should be identical to the application being modeled. This ratio is defined as the surface area of one member of the couple divided
by the surface area of the other member of the couple. Only the area in contact with the electrolyte (wetted area) is used in this
calculation. In low-resistivity electrolytes, maintaining proximity between the materials being coupled may be more important than
maintaining the exact area ratio. Also, with some couples, such as copper coupled to aluminum, there may be effects of corrosion
products washing from one electrode to another which may have to be considered in determining specimen placement.
4.2.2 Laboratory tests are normally performed on rectangular plates or on cylinders. When modeling service applications, the
shapes of the couple members should approximate the shapes in the application. Frequently complex shapes are simplified for
testing purposes. The shape of the specimen is more important in electrolytes of low conductivity, where voltage drop in the
electrolyte is significant. In highly conductive electrolytes, the shapes of the couple members may therefore deviate somewhat from
the shapes in the application.
4.3 Specimen Preparation:
4.3.1 The edges of the test specimens should be prepared so as to eliminate all sheared or cold-worked metal except that
cold-working introduced by stamping for identification. Shearing will, in some cases, cause considerable attack. Therefore,
specimens having sheared edges should not be used. The edges should be finished by machining or polishing. The slight amount
of cold working resulting from machining will not introduce any serious error.
4.3.2 Specimens should be cleaned in accordance with Practice G1, or else the specimen surface condition should be similar
to the application being modeled. The metallurgical condition of the specimens should be similar to the application being modeled.
In all cases surface contamination, such as dirt, grease, oil, and thick oxides, should be removed prior to weighing and exposure
to the test environment.
4.3.3 The specimen identification system must be one that will endure throughout the test period. Edge notches, drilled holes,
stamped numbers, and tags are some of the methods used for identification. The identification system must not induce corrosion
attack in any way.
4.4 Number of Specimens:
4.4.1 The number of galvanic couples to be tested will be determined by whether or not one or more periodic specimen removals
are scheduled during the course of the test. As a minimum, duplicate and preferably triplicate specimens should be tested for any
given test period to determine the variability in the galvanic corrosion behavior. The effect of the number of replications on the
application of the results is set forth in Guide G16.
4.4.2 Control specimens should also be tested to provide corrosion rates of the individual metals and alloys without coupling
for comparisons. These specimens should be of the same alloys, shapes, sizes, and metallurgical conditions as the materials in the
couple.
5. Test Environment
5.1 Laboratory Tests:
5.1.1 In the laboratory, the test solution should closely approximate the service environment. The amount of test solution used
3 2
depends on the size of the test specimens. A good rule of thumb is to use 40 cm of test solution for every 1 cm of exposed surface
area of both members of the couple. The volume of test solution may be varied to closely approximate the service application.
5.1.2 Galvanic corrosion tests conducted for an extensive period of time may exhaust important constituents of the original
solution. Some accumulated corrosion products may act as corrosion accelerators or inhibitors. These variables may greatly change
the end results, and replenishment of the solution should be chosen to be representative of the service application. A test system
using continuously replenished test electrolytes is often the only solution to this problem.
5.1.3 Periodic measurements of the test environment should be made when the test duration in a fixed volume solution is for
periods of several days or longer. These observations may include temperature, pH, O , H S, CO , NH , conductivity, and pertinent
2 2 2 3
metal ion content.
5.2 Field Tests—Field testing should be performed in an environment similar to the service environment. Periodic measurements
of those environmental variables which could vary with time, such as temperature, dissolved O , and so forth, should be made.
6. Procedure
6.1 Laboratory Versus Field Testing:
6.1.1 Galvanic corrosion tests are conducted in the laboratory for several purposes: (1) inexpensive screening to reduce
expensive field testing, (2) study of the effects of environmental variables, and (3) stu
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.