ASTM G71-81(2019)
(Guide)Standard Guide for Conducting and Evaluating Galvanic Corrosion Tests in Electrolytes
Standard Guide for Conducting and Evaluating Galvanic Corrosion Tests in Electrolytes
SIGNIFICANCE AND USE
3.1 Use of this guide is intended to provide information on the galvanic corrosion of metals in electrical contact in an electrolyte that does not have a flow velocity sufficient to cause erosion-corrosion or cavitation.
3.2 This standard is presented as a guide for conducting galvanic corrosion tests in liquid electrolyte solutions, both in the laboratory and in service environments. Adherence to this guide will aid in avoiding some of the inherent difficulties in such testing.
SCOPE
1.1 This guide covers conducting and evaluating galvanic corrosion tests to characterize the behavior of two dissimilar metals in electrical contact in an electrolyte under low-flow conditions. It can be adapted to wrought or cast metals and alloys.
1.2 This guide covers the selection of materials, specimen preparation, test environment, method of exposure, and method for evaluating the results to characterize the behavior of galvanic couples in an electrolyte.
Note 1: Additional information on galvanic corrosion testing and examples of the conduct and evaluation of galvanic corrosion tests in electrolytes are given in Refs (1)2 through (2).
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: G71 − 81 (Reapproved 2019)
Standard Guide for
Conducting and Evaluating Galvanic Corrosion Tests in
Electrolytes
ThisstandardisissuedunderthefixeddesignationG71;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope G1 Practice for Preparing, Cleaning, and Evaluating Corro-
sion Test Specimens
1.1 This guide covers conducting and evaluating galvanic
G3 Practice for Conventions Applicable to Electrochemical
corrosion tests to characterize the behavior of two dissimilar
Measurements in Corrosion Testing
metals in electrical contact in an electrolyte under low-flow
G4 Guide for Conducting Corrosion Tests in Field Applica-
conditions. It can be adapted to wrought or cast metals and
tions
alloys.
G16 Guide for Applying Statistics to Analysis of Corrosion
1.2 This guide covers the selection of materials, specimen
Data
preparation,testenvironment,methodofexposure,andmethod
G31 Guide for Laboratory Immersion Corrosion Testing of
for evaluating the results to characterize the behavior of
Metals
galvanic couples in an electrolyte.
G46 Guide for Examination and Evaluation of Pitting Cor-
rosion
NOTE 1—Additional information on galvanic corrosion testing and
examples of the conduct and evaluation of galvanic corrosion tests in
3. Significance and Use
electrolytes are given in Refs (1) through (2).
1.3 The values stated in SI units are to be regarded as
3.1 Use of this guide is intended to provide information on
standard. No other units of measurement are included in this the galvanic corrosion of metals in electrical contact in an
standard.
electrolytethatdoesnothaveaflowvelocitysufficienttocause
erosion-corrosion or cavitation.
1.4 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
3.2 This standard is presented as a guide for conducting
responsibility of the user of this standard to establish appro-
galvanic corrosion tests in liquid electrolyte solutions, both in
priate safety, health, and environmental practices and deter-
the laboratory and in service environments. Adherence to this
mine the applicability of regulatory limitations prior to use.
guide will aid in avoiding some of the inherent difficulties in
1.5 This international standard was developed in accor-
such testing.
dance with internationally recognized principles on standard-
4. Test Specimens
ization established in the Decision on Principles for the
Development of International Standards, Guides and Recom-
4.1 Material—Test specimens should be manufactured from
mendations issued by the World Trade Organization Technical
thesamematerialasthoseusedintheserviceapplicationbeing
Barriers to Trade (TBT) Committee.
modeled. Minor compositional or processing differences be-
tween materials or between different heats can greatly affect
2. Referenced Documents
the results in some cases.
2.1 ASTM Standards:
4.2 Size and Shape:
4.2.1 Thesizeandshapeofthetestspecimensaredependent
on restrictions imposed by the test location. When determining
This guide is under the jurisdiction ofASTM Committee G01 on Corrosion of
material behavior in the laboratory, it is advisable to use the
Metals and is the direct responsibility of Subcommittee G01.11 on Electrochemical
Measurements in Corrosion Testing.
largest specimens permissible within the constraints of the test
Current edition approved May 1, 2019. Published June 2019. Originally
equipment.Ingeneral,theratioofsurfaceareatometalvolume
approved in 1981. Last previous edition approved in 2014 as G71 – 81 (2014). DOI:
should be large in order to favor maximum corrosion loss per
10.1520/G0071-81R19.
weight. Sufficient thickness should be employed, however, to
The boldface numbers in parentheses refer to a list of references at the end of
this standard.
minimize the possibility of perforation of the specimens during
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
the test exposure. When modeling large components, the size
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
of the specimens should be as large as practical. When
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. modeling smaller components, specimen size should be as
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G71 − 81 (2019)
close as possible to that of the application being modeled. 5. Test Environment
Surface area ratio in the test should be identical to the
5.1 Laboratory Tests:
application being modeled. This ratio is defined as the surface
5.1.1 In the laboratory, the test solution should closely
area of one member of the couple divided by the surface area
approximate the service environment. The amount of test
of the other member of the couple. Only the area in contact
solutionuseddependsonthesizeofthetestspecimens.Agood
with the electrolyte (wetted area) is used in this calculation. In
3 2
rule of thumb is to use 40 cm of test solution for every 1 cm
low-resistivity electrolytes, maintaining proximity between the
of exposed surface area of both members of the couple. The
materials being coupled may be more important than maintain-
volume of test solution may be varied to closely approximate
ing the exact area ratio. Also, with some couples, such as
the service application.
copper coupled to aluminum, there may be effects of corrosion
5.1.2 Galvanic corrosion tests conducted for an extensive
products washing from one electrode to another which may
period of time may exhaust important constituents of the
have to be considered in determining specimen placement.
original solution. Some accumulated corrosion products may
4.2.2 Laboratory tests are normally performed on rectangu-
act as corrosion accelerators or inhibitors.These variables may
lar plates or on cylinders.When modeling service applications,
greatly change the end results, and replenishment of the
the shapes of the couple members should approximate the
solution should be chosen to be representative of the service
shapes in the application. Frequently complex shapes are
application. A test system using continuously replenished test
simplified for testing purposes. The shape of the specimen is
electrolytes is often the only solution to this problem.
more important in electrolytes of low conductivity, where
5.1.3 Periodic measurements of the test environment should
voltage drop in the electrolyte is significant. In highly conduc-
bemadewhenthetestdurationinafixedvolumesolutionisfor
tive electrolytes, the shapes of the couple members may
periods of several days or longer. These observations may
therefore deviate somewhat from the shapes in the application.
includetemperature,pH,O ,H S,CO ,NH ,conductivity,and
2 2 2 3
pertinent metal ion content.
4.3 Specimen Preparation:
4.3.1 The edges of the test specimens should be prepared so
5.2 Field Tests—Field testing should be performed in an
as to eliminate all sheared or cold-worked metal except that
environment similar to the service environment. Periodic
cold-working introduced by stamping for identification. Shear-
measurements of those environmental variables which could
ing will, in some cases, cause considerable attack. Therefore,
varywithtime,suchastemperature,dissolvedO ,andsoforth,
specimens having sheared edges should not be used.The edges
should be made.
should be finished by machining or polishing. The slight
amount of cold working resulting from machining will not
6. Procedure
introduce any serious error.
6.1 Laboratory Versus Field Testing:
4.3.2 Specimens should be cleaned in accordance with
6.1.1 Galvanic corrosion tests are conducted in the labora-
Practice G1, or else the specimen surface condition should be
tory for several purposes: (1) inexpensive screening to reduce
similar to the application being modeled. The metallurgical
expensive field testing, (2) study of the effects of environmen-
condition of the specimens should be similar to the application
tal variables, and (3) study of the corrosion accelerating or
being modeled. In all cases surface contamination, such as dirt,
protective effects of various anode/cathode surface area ratios.
grease, oil, and thick oxides, should be removed prior to
6.1.2 The materials proven in the laboratory to be the most
weighing and exposure to the test environment.
promising should also be tested in the field, since it is
4.3.3 The specimen identification system must be one that
frequently impossible to duplicate the actual service environ-
will endure throughout the test period. Edge notches, drilled
ment in the laboratory.
holes, stamped numbers, and tags are some of the methods
6.2 Test Procedure:
used for identification. The identification system must not
induce corrosion attack in any way. 6.2.1 Specimens should be electrically joined before expo-
sure.Thereareanumberofmethodsforjoiningthespecimens.
4.4 Number of Specimens:
Laboratory testing generally employs external electrical con-
4.4.1 The number of galvanic couples to be tested will be
nection through wires such as to allow current measurement
determined by whether or not one or more periodic specimen
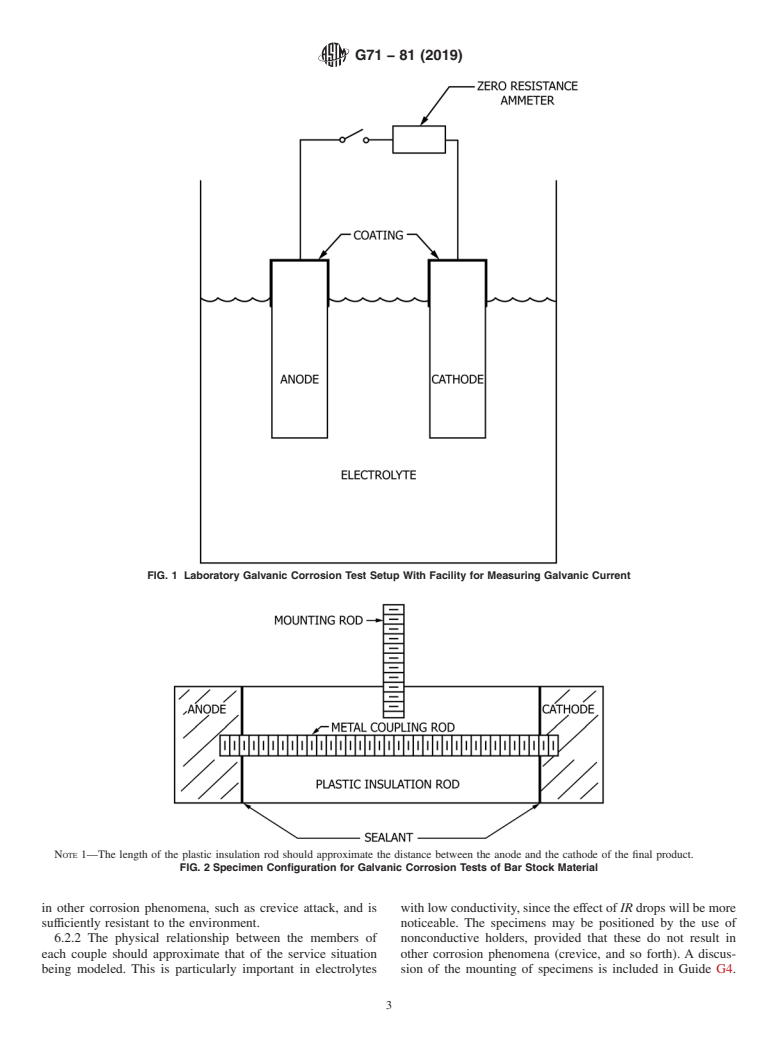
(see Fig. 1)
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.