ASTM D5011-92(2009)
(Practice)Standard Practices for Calibration of Ozone Monitors Using Transfer Standards
Standard Practices for Calibration of Ozone Monitors Using Transfer Standards
SIGNIFICANCE AND USE
The reactivity and instability of O3 precludes the storage of O3 concentration standards for any practical length of time, and precludes direct certification of O3 concentrations as SRM's. Moreover, there is no available SRM that can be readily and directly adapted to the generation of O3 standards analogous to permeation devices and standard gas cylinders for sulfur dioxide and nitrogen oxides. Dynamic generation of O3 concentrations is relatively easy with a source of ultraviolet (UV) radiation. However, accurately certifying an O3 concentration as a primary standard requires assay of the concentration by a comprehensively specified analytical procedure, which must be performed every time a standard is needed.
The primary UV standard photometers, which are usually used at a fixed location under controlled conditions, are used to certify transfer standards that are then transported to the field sites where the ambient ozone monitors are being used. See Practice D 5110.
The advantages of this procedure are:
All O3 monitors in a given network or region may be traced to a single primary standard.
The primary standard is used at only one location, under controlled conditions.
Transfer standards are more rugged and more easily portable than primary standards.
Transfer standards may be used to intercompare various primary standards.
SCOPE
1.1 These practices describe means for calibrating ambient, workplace or indoor ozone monitors, using transfer standards.
1.2 These practices describe five types of transfer standards:
(A) Analytical instruments
(B) Boric acid potassium iodide (BAKI) manual analytical procedure
(C) Gas phase titration with excess nitric oxide
(D) Gas phase titration with excess ozone
(E) Ozone generator device.
1.3 These practices describe procedures to establish the authority of transfer standards: qualification, certification, and periodic recertification.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. See Section 8 for specific precautionary statements.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D5011 − 92 (Reapproved 2009)
Standard Practices for
Calibration of Ozone Monitors Using Transfer Standards
This standard is issued under the fixed designation D5011; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope CertificationofOzoneTransferStandardsUsingUltravio-
let Photometry
1.1 These practices describe means for calibrating ambient,
E591Practice for Safety and Health Requirements Relating
workplace or indoor ozone monitors, using transfer standards.
to Occupational Exposure to Ozone (Withdrawn 1990)
1.2 Thesepracticesdescribefivetypesoftransferstandards:
2.2 Other Documents:
(A) Analytical instruments
40CFR Part 50, Environmental Protection Agency Regula-
(B) Boric acid potassium iodide (BAKI) manual analytical 4
tions on Ambient Air Monitoring Reference Methods
procedure
(C) Gas phase titration with excess nitric oxide
3. Terminology
(D) Gas phase titration with excess ozone
3.1 For definitions of terms used in this standard, see
(E) Ozone generator device.
Terminology D1356.
1.3 These practices describe procedures to establish the
3.2 Definitions of Terms Specific to This Standard:
authority of transfer standards: qualification, certification, and
3.2.1 primary standard—a standard directly defined and
periodic recertification.
established by some authority, against which all secondary
1.4 This standard does not purport to address all of the
standards are compared.
safety concerns, if any, associated with its use. It is the
3.2.2 secondary standard—a standard used as a means of
responsibility of the user of this standard to establish appro-
comparison, but checked against a primary standard.
priate safety and health practices and determine the applica-
3.2.3 standard—an accepted reference sample or device
bility of regulatory limitations prior to use. See Section 8 for
used for establishing measurement of a physical quantity.
specific precautionary statements.
3.2.4 transfer standard—a type of secondary standard. It is
2. Referenced Documents
a transportable device or apparatus, which, together with
operational procedures, is capable of reproducing pollutant
2.1 ASTM Standards:
concentration or producing acceptable assays of pollutant
D1071Test Methods for Volumetric Measurement of Gas-
concentrations.
eous Fuel Samples
D1193Specification for Reagent Water
3.2.5 zero air—purified air that does not contain ozone and
D1356Terminology Relating to Sampling and Analysis of
does not contain any other component that may interfere with
Atmospheres
the measurement. See 7.1.
D3195Practice for Rotameter Calibration
3.3 Symbols:
D3249Practice for General Ambient Air Analyzer Proce-
dures
b = Spectrophotometer cell path length, cm. See
D3631Test Methods for Measuring Surface Atmospheric
Annex A2.
Pressure d = Average of discrete single point comparisons.
avg
D5110Practice for Calibration of Ozone Monitors and
See Annex A1.
d = Single point comparison. See Annex A1.
i
F = Diluent air flow, mL/min.
D
These practices are under the jurisdiction of ASTM Committee D22 on Air
F ' = New diluent air flow, mL/min.
D
Quality and are the direct responsibility of Subcommittee D22.03 on Ambient
F = NO flow, mL/min.
NO
Atmospheres and Source Emissions.
Current edition approved March 1, 2009. Published March 2009. Originally
approved in 1989. Last previous edition approved in 2003 as D5011–92 (2003).
DOI: 10.1520/D5011-92R09. The last approved version of this historical standard is referenced on
For referenced ASTM standards, visit the ASTM website, www.astm.org, or www.astm.org.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM AvailablefromU.S.GovernmentPrintingOfficeSuperintendentofDocuments,
Standards volume information, refer to the standard’s Document Summary page on 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401, http://
the ASTM website. www.access.gpo.gov.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D5011 − 92 (2009)
F = Flow through the O generator, mL/min. s = Standard deviation of single point comparisons.
O 3 d
F = Flowrate corrected to reference conditions
See Annex A1.
R
(25°C and 101.3 kPa), mL/min. See AnnexA2. s = Relativestandarddeviationofthesixintercepts.
i
F = Flowrate at sampling conditions, mL/min. See
See Annex A1.
S
Annex A2. s = Relative standard deviation of the six slopes.
m
F = The total flow required at the output manifold
See Annex A1.
T
(monitors demand plus 10 to 50% excess), t = Residence time in reaction chamber, min.
R
mL/min. t = Sampling time, min. See Annex A2.
s
T = Temperature at sampling conditions, °C. See
I = The intensity of light which passes through the
S
Annex A2
photometer absorption cell and is sensed by the
URL = Upper range limit of O or NO monitor, ppm.
detector when the cell contains an O sample.
V = Volume of I solution, mL. See Annex A2
See Annex A4. i 2
VO = Volume of O absorbed, µL. See Annex A2.
[I ] = ConcentrationofeachI standard,molI /L.See 3 3
2 i 2 2
V = Volume of air sampled, corrected to 25°C and
R
Annex A2.
101.3 kPa (1 atm), mL. See Annex A2.
I = Average intercept. See Annex A1.
avg
V = Volume of the reaction chamber, mL.
I = Individual intercepts. See Annex A1. RC
i
y =O concentration indicated by the transfer
I = The intensity of light which passes through the i 3
O
standard, ppm. See 10.6.2.
photometer absorption cell and is sensed by the
Z = Recorder response with zero air, % scale.
detector when the cell contains zero air. See
Annex A4.
4. Summary of Practices
m = Average slope. See Annex A1.
avg
4.1 These practices describe the procedures necessary to
m = Individual slopes. See Annex A1.
i
establish the authority of ozone transfer standards:
mol I =I released, mols. See Annex A2.
2 2
qualification, certification, and periodic recertification. Quali-
N = Normality of KIO , equivalent/L. See Annex
KIO 3
fication consists of demonstrating that a candidate transfer
A2.
standard is sufficiently stable (repeatable) to be useful as a
[NO] = Diluted NO concentration, ppm. See AnnexA4.
[NO] = Original NO concentration, ppm. See Annex transfer standard. Repeatability is necessary over a range of
ORIG
variables (such as temperature, line voltage, barometric
A3.
[NO] = HighestNOconcentrationrequiredattheoutput
pressure, elapsed time, operator adjustments, relocation, etc.),
OUT
manifold, ppm. It is approximately equal to any of which may be encountered during use of the transfer
90% of the upper range limit of the O concen-
standard. Tests and possible compensation techniques for
tration to be determined. See Annex A3. several such common variables are described. Detailed certi-
[NO] = NO concentration (approximate) in the reaction
fication procedures are provided, and the quantitative specifi-
RC
chamber, ppm. See Annex A3.
cations necessary to maintain continuous certification of the
[NO] = NO concentration remaining after addition of
REM transfer standard are also provided.
O , ppm. See Annex A3.
4.2 Method A—A dedicated ozone monitor is tested as
[NO] = Concentration of the undiluted NO standard,
STD
described in 4.1 to demonstrate its authority as a transfer
ppm.
standard.
n = Number of comparisons. See Eq 4
[O ] = Certified O concentration, ppm. 4.3 Method B—This method (1) is based on the reaction
3 CERT 3
[O ] = Diluted certified O concentration, ppm.
between ozone (O ) and potassium iodide (KI) to release
3 CERT' 3
[O ] =O concentration produced by the O generator,
iodine (I ) in accordance with the following stoichiometric
3 GEN 3 3
ppm. See Annex A4.
equation (2):
[O ] = Indicated O concentration, ppm. See Annex
3 OUT 3
2 1
O 12I 12H 5 I 1H O1O (1)
3 2 2 2
A2.
[O ] = Diluted O concentration, ppm. The stoichiometry is such that the amount of I released is
3 OUT' 3 2
[O ] =O concentration (approximate) at the output
equaltotheamountofO absorbed.Ozoneisabsorbedina0.1
3 RC 3
manifold, ppm.
N boric acid solution containing 1% KI, and the I released
− −
P = Vapor pressure of HOat T , kPa, wet volume
H O 2 S reacts with excess iodide ion (I ) to form triiodide ion (I ),
standard. (For a dry standard, P =0.) (See
H O which is measured spectrophotometrically at a wavelength of
Test Method D4230 for tables of saturation
352 nm. The output of a stable O generator is assayed in this
vapor pressure of water.) See Annex A2.
manner, and the O generator is immediately used to calibrate
P = Dynamic specification, determined empirically,
R the O monitor.
to ensure complete reaction of O or NO,
4.4 Method C—This procedure is based on the rapid gas
ppm/min.
phase reaction between nitric oxide (NO) and O , as described
P = Barometric pressure at sampling conditions,
S
by the following equation (3):
kPa. See Annex A2.
S = Slope of KI calibration curve, mL/mol/cm. See
c
The boldface numbers in parentheses refer to the references at the end of these
Annex A2.
practices.
D5011 − 92 (2009)
NO1O 5 NO1O (2) 5. Significance and Use
3 2
5.1 ThereactivityandinstabilityofO precludesthestorage
When O is added to excess NO in a dynamic system, the 3
of O concentration standards for any practical length of time,
decrease in NO response is equivalent to the concentration of
and precludes direct certification of O concentrations as
O added. The NO is obtained from a standard NO cylinder, 3
SRM’s. Moreover, there is no available SRM that can be
and the O is produced by a stable O generator. A chemilu-
3 3
readily and directly adapted to the generation of O standards
minescence NO analyzer is used to measure the change in NO 3
analogoustopermeationdevicesandstandardgascylindersfor
concentration.TheconcentrationofO addedmaybevariedto
sulfur dioxide and nitrogen oxides. Dynamic generation of O
obtain calibration concentrations over the range desired. The 3
concentrations is relatively easy with a source of ultraviolet
dynamic system is designed to produce locally high concen-
(UV) radiation. However, accurately certifying an O concen-
trationsofNOandO inthereactionchamber,withsubsequent 3
tration as a primary standard requires assay of the concentra-
dilution, to effect complete O reaction with relatively small
tion by a comprehensively specified analytical procedure,
chamber volumes.
which must be performed every time a standard is needed.
4.5 Method D—This procedure is based on the rapid gas
5.2 The primary UV standard photometers, which are usu-
phase reaction between O and nitric oxide (NO) as described
ally used at a fixed location under controlled conditions, are
by the following equation (3):
usedtocertifytransferstandardsthatarethentransportedtothe
NO1O 5 NO 1O (3)
3 2 2
field sites where the ambient ozone monitors are being used.
See Practice D5110.
When NO is added to excess O in a dynamic system, the
decrease in O response observed on an uncalibrated O
3 3
5.3 The advantages of this procedure are:
monitor is equivalent to the concentration of NO added. By
5.3.1 All O monitors in a given network or region may be
measuring this decrease in response and the initial response,
traced to a single primary standard.
the O concentration can be determined. Additional O con-
3 3 5.3.2 The primary standard is used at only one location,
centrations are generated by dilution. The gas phase titration
under controlled conditions.
(GPT) system is used under predetermined flow conditions to
5.3.3 Transfer standards are more rugged and more easily
insure that the reaction of NO is complete and that further
portable than primary standards.
reaction of the resultant nitrogen dioxide (NO ) with residual
2 5.3.4 Transfer standards may be used to intercompare vari-
O is negligible.
ous primary standards.
4.6 Method E—A dedicated ozone generator is tested as
6. Apparatus
described in 4.1 to demonstrate its authority as a transfer
standard. 6.1 Apparatus Common to Methods A Through E:
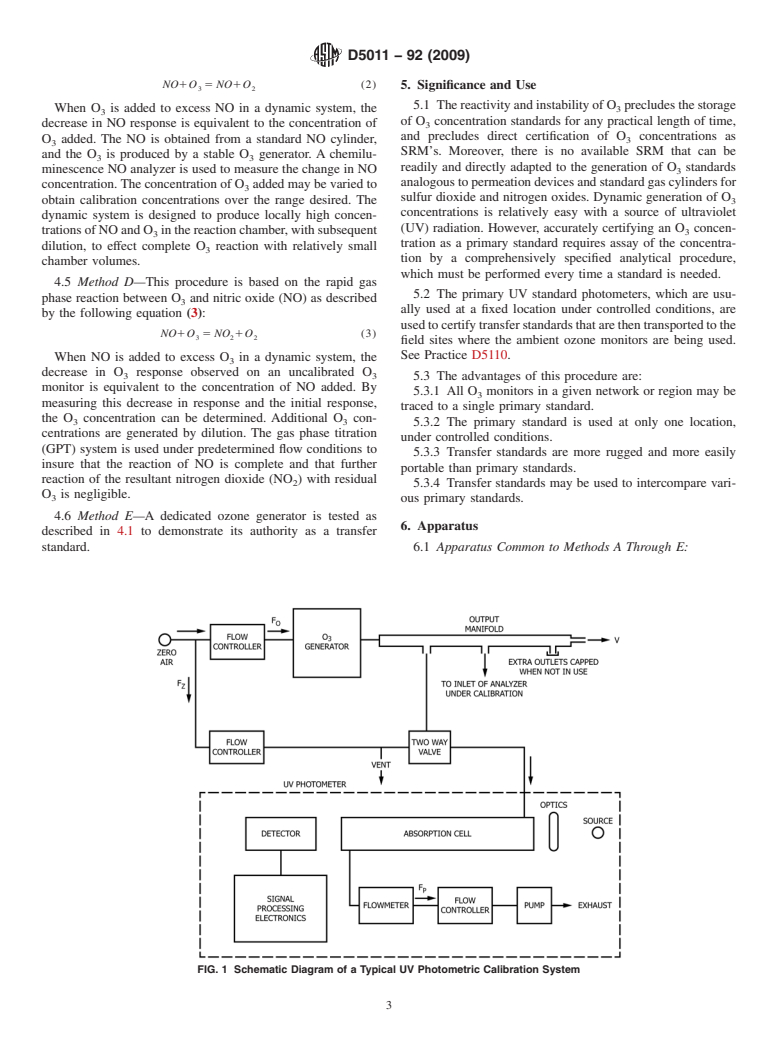
FIG. 1 Schematic Diagram of a Typical UV Photometric Calibration System
D5011 − 92 (2009)
6.1.1 UV Photometric calibration system, as shown in Fig. 6.1.1.7 Barometer or Pressure Indicator—accurate to 6250
1, consisting of the following: Pa (2 Torr). The barometer or pressure indicator is used to
measure the pressure of the gas in the cell in order to calculate
6.1.1.1 Primary Ozone Standard—a UV photometer, con-
a pressure correction. Most photometer cells operate at atmo-
sisting of a low-pressure mercury discharge lamp, collimation
spheric pressure. If there are no restrictions between the cell
optics (optional), an absorption cell, a detector, and signal-
andtheoutputmanifold,thecellpressureshouldbeverynearly
processing electronics. It shall be capable of measuring the
the same as the local barometric pressure. A certified local
transmittance, I/I , at a wavelength of 253.7 nm with sufficient
barometric pressure reading can then be used for the pressure
precision that the standard deviation of the concentration
correction. If the cell pressure is different than the local
measurementsdoesnotexceedthegreaterof0.005ppmor3%
barometric pressure, some means of accurately measuring the
of the concentration. It shall incorporate means to assure that
cell pressure (manometer, pressure gage, or pressure trans-
noO isgeneratedinthecellbytheUVlamp.Thisisgenerally
ducer) is required. This device shall be calibrated against a
accomplishedbyfilteringoutthe184.9nmHglinewithahigh
suitable pressure standard, in accordance with Test Methods
silica filter. In addition, at least 99.5% of the radiation sensed
D3631.
bythedetectorshallbe253.7nm.Thisisusuallyaccomplished
6.1.2 Output Indicating Device, such as Continuous Strip
by using a solar blind photodiode tube. The length of the light
Chart Recorder or Digital Volt Meter—If a recorder is used, it
path through the absorption cell shall be known with an
shall have the following specifications:
accuracy within at least 99.5%. In addition the cell and
Accuracy ±0.25 % of span
associated plumbing shall be designed to minimize loss of O
Chart width no less than 150 mm
from contact with surfaces (4). See Practice D5110.
Time for full-scale travel 1 s
6.1.1.2 Air Flow Controller—capableofregulatingairflows
6.1.2.1 If a digital voltmeter is used, it shall have an
as necessary to meet the output stability and photometer
accuracy of 60.25% of range.
precision requirements.
6.1.2.2 Method A output indicating device shall be consid-
6.1.1.3 Flowmeters—calibrat
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.