ASTM E355-96(2014)
(Practice)Standard Practice for Gas Chromatography Terms and Relationships
Standard Practice for Gas Chromatography Terms and Relationships
ABSTRACT
This practice presents the terms, parameters, symbols, units, and relationships used in gas elution chromatography. Most of the terms described herein should also apply to other kinds of gas chromatography and various liquid column chromatographic techniques. At this time, however, they are not standardized for the latter usage. These terms include names of techniques, apparatuses and reagents, parameters used in data recording and presentation of isothermal retention data, and retention parameters.
SCOPE
1.1 This practice covers primarily the terms and relationships used in gas elution chromatography. However, most of the terms should also apply to other kinds of gas chromatography and are also valid in the various liquid column chromatographic techniques, although at this time they are not standardized for the latter usage.
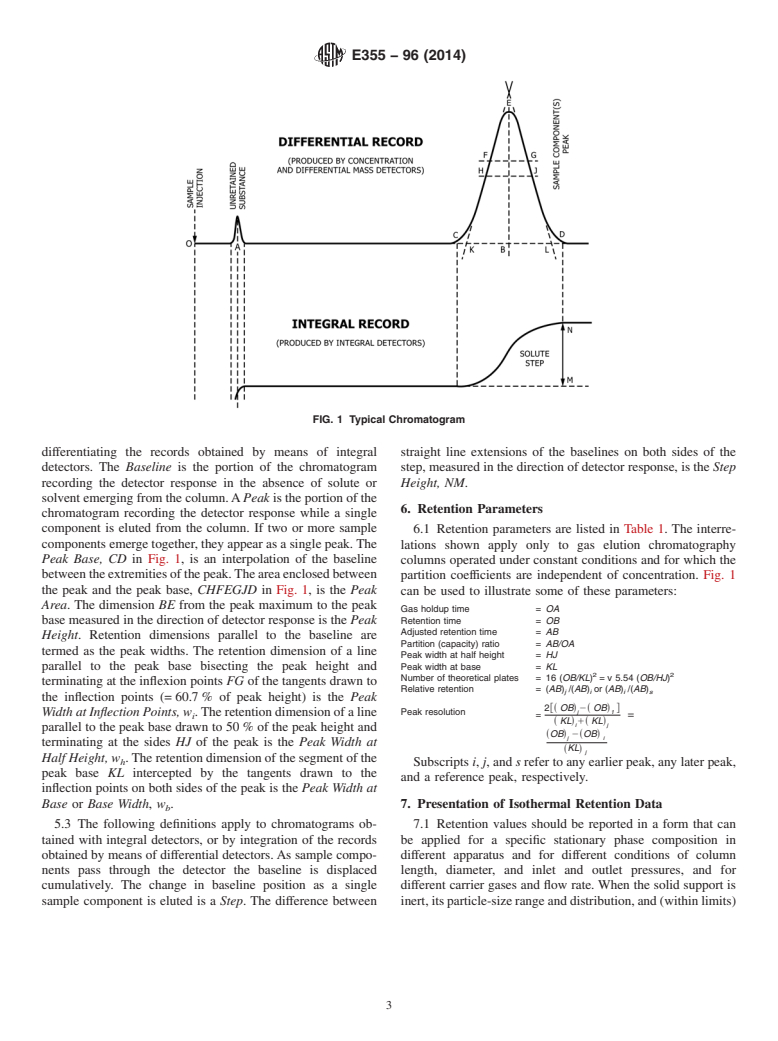
General Information
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E355 − 96 (Reapproved 2014)
Standard Practice for
1
Gas Chromatography Terms and Relationships
This standard is issued under the fixed designation E355; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope 2.6 Gas-Displacement Chromatography employs a desor-
bent as the carrier gas or in the carrier gas to displace a less
1.1 This practice covers primarily the terms and relation-
strongly held solute from the stationary phase which in turn
ships used in gas elution chromatography. However, most of
displaces the next less strongly held one etc., causing the
the terms should also apply to other kinds of gas chromatog-
components to emerge in the normal order, that is, least-to-
raphy and are also valid in the various liquid column chro-
most strongly absorbed.
matographic techniques, although at this time they are not
standardized for the latter usage. 2.7 Isothermal Gas Chromatography is the version of the
technique in which the column temperature is held constant
2. Names of Techniques
during the passage of the sample components through the
separation column.
2.1 Gas Chromatography, abbreviated as GC, comprises all
chromatographic methods in which the moving phase is
2.8 Programmed Temperature Gas Chromatography
gaseous. The stationary phase may be either a dry granular
(PTGC), is the version of the technique in which the column
solid or a liquid supported by the granules or by the wall of the
temperature is changed with time during the passage of the
column, or both. Separation is achieved by differences in the
sample components through the separation column. In linear
distribution of the components of a sample between the mobile
PTGC the program rate is constant during analysis. Isothermal
and stationary phases, causing them to move through the
intervals may be included in the temperature program.
column at different rates and from it at different times. In this
2.9 Programmed Flow, Pressure, or Velocity Gas Chroma-
recommended practice gas elution chromatography is implied.
tographyistheversionofthetechniqueinwhichthecarriergas
2.2 Gas-Liquid Chromatography, abbreviated as GLC, uti-
flow, pressure, or velocity is changed during analysis.
lizesaliquidasthestationaryphase,whichactsasasolventfor
2.10 Reaction Gas Chromatography is the version of the
the sample components.
technique in which the composition of the sample is changed
2.3 Gas-Solid Chromatography, abbreviated as GSC, uti-
between sample introduction and the detector.The reaction can
lizes an active solid (adsorbent) as the stationary phase.
take place upstream of the column when the chemical compo-
2.4 Gas Elution Chromatography utilizes a continuous inert sition of the individual components passing through the col-
umn differs from that of the original sample, or between the
gas flow as the carrier gas and the sample is introduced as a gas
or a liquid with a finite volume into the carrier gas stream. If column and the detector when the original sample components
are separated in the column but their chemical composition is
the sample is introduced as a liquid, it is vaporized in the
system prior to or during passage through the separation changed prior to entering the detection device.
column.
2.11 Pyrolysis Gas Chromatography is the version of reac-
2.5 Gas-Frontal Chromatography is a technique in which a tion gas chromatography in which the original sample is
decomposed by heat to more volatile components prior to
continuous stream of carrier gas mixed with sample vapor is
instantaneously replaced by a continuous stream of carrier gas passage through the separation column.
containing sample vapor at a different concentration. The
3. Apparatus
concentration profile is therefore step-shaped at the column
inlet.
3.1 Sample Inlet Systems, represent the means for introduc-
ing samples into the separation column, including the heated
1
zones permitting the vaporization of the introduced liquid
This practice is under the jurisdiction of ASTM Committee E13 on Molecular
Spectroscopy and Separation Science and is the direct responsibility of Subcom-
samples prior to their passage through the column. Sample
mittee E13.19 on Separation Science.
introduction can be carried out by introduction of a liquid,
Current edition approved May 1, 2014. Published June 2014. Originally
solid, or gas into the carrier-gas stream. The sample may be
approved in 1968. Last previous edition approved in 2007 as E355 – 96 (2007).
DOI: 10.1520/E0355-96R14. vaporized b
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.