ASTM D3561-16(2021)e1
(Test Method)Standard Test Method for Lithium, Potassium, and Sodium Ions in Brackish Water, Seawater, and Brines by Atomic Absorption Spectrophotometry
Standard Test Method for Lithium, Potassium, and Sodium Ions in Brackish Water, Seawater, and Brines by Atomic Absorption Spectrophotometry
SIGNIFICANCE AND USE
5.1 Identification of a brackish water, seawater, or brine is determined by comparison of the concentrations of their dissolved constituents. The results are used to evaluate the water as a possible pollutant, or as a commercial source of a valuable constituent such as lithium.
SCOPE
1.1 This test method covers the determination of soluble lithium, potassium, and sodium ions in brackish water, seawater, and brines by atomic absorption spectrophotometry.2
1.2 Samples containing from 0.1 to 70 000 mg/L of lithium, potassium, and sodium may be analyzed by this test method.
1.3 This test method has been used successfully with artificial brine samples. It is the user's responsibility to ensure the validity of this test method for waters of untested matrices.
1.4 The values stated in SI units are to be regarded as standard. The values given in parentheses are mathematical conversion to inch-pound units that are provided for information only and are not considered standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
´1
Designation: D3561 − 16 (Reapproved 2021)
Standard Test Method for
Lithium, Potassium, and Sodium Ions in Brackish Water,
Seawater, and Brines by Atomic Absorption
Spectrophotometry
This standard is issued under the fixed designation D3561; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
ε NOTE—The WTO caveat was editorially added in November 2021.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This test method covers the determination of soluble
D1129 Terminology Relating to Water
lithium, potassium, and sodium ions in brackish water,
D1193 Specification for Reagent Water
seawater, and brines by atomic absorption spectrophotometry.
D2777 Practice for Determination of Precision and Bias of
1.2 Samplescontainingfrom0.1to70 000mg/Loflithium,
Applicable Test Methods of Committee D19 on Water
potassium, and sodium may be analyzed by this test method.
D3370 Practices for Sampling Water from Flowing Process
Streams
1.3 This test method has been used successfully with
D5810 Guide for Spiking into Aqueous Samples
artificial brine samples. It is the user’s responsibility to ensure
D5847 Practice for Writing Quality Control Specifications
the validity of this test method for waters of untested matrices.
for Standard Test Methods for Water Analysis
1.4 The values stated in SI units are to be regarded as
standard. The values given in parentheses are mathematical
3. Terminology
conversion to inch-pound units that are provided for informa-
3.1 Definitions:
tion only and are not considered standard.
3.1.1 For definitions of terms used in this standard, refer to
1.5 This standard does not purport to address all of the
Terminology D1129.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
4. Summary of Test Method
priate safety, health, and environmental practices and deter-
4.1 This test method is dependent on the fact that metallic
mine the applicability of regulatory limitations prior to use.
elements, in the ground state, will absorb light of the same
1.6 This international standard was developed in accor-
wavelength they emit when excited. When radiation from a
dance with internationally recognized principles on standard-
given excited element is passed through a flame containing
ization established in the Decision on Principles for the
ground state atoms of that element, the intensity of the
Development of International Standards, Guides and Recom-
transmitted radiation will decrease in proportion to the amount
mendations issued by the World Trade Organization Technical
of ground state element in the flame. A hollow cathode lamp
Barriers to Trade (TBT) Committee.
whose cathode is made of the element to be determined
4,5
provides the radiation. The metal atoms to be measured are
placed in the beam of radiation by aspirating the specimen into
This test method is under the jurisdiction of ASTM Committee D19 on Water
and is the direct responsibility of Subcommittee D19.05 on Inorganic Constituents
in Water.
Current edition approved Nov. 1, 2021. Published December 2021. Originally For referenced ASTM standards, visit the ASTM website, www.astm.org, or
approved in 1977. Last previous edition approved in 2016 as D3561 – 16. DOI: contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
10.1520/D3561-16R21E01. Standards volume information, refer to the standard’s Document Summary page on
Fletcher, G. F. and Collins, A. G., Atomic Absorption Methods of Analysis of the ASTM website.
Oilfield Brines: Barium, Calcium, Copper, Iron, Lead, Lithium, Magnesium, Angino, E. E., and Billings, G. K., Atomic Absorption Spectrophotometry in
Manganese, Potassium, Sodium, Strontium, and Zinc. U.S. Bureau of Mines, Report Geology, Elsevier Publishing Co., New York, NY, 1967.
ofInvestigations7861,1974,14pp.,Collins,A.G.GeochemistryofOilfieldWaters, Dean, J. A., and Rains, T. C., Editors, Flame Emission and Atomic Absorption
Elsevier, New York, NY, 1975. Spectrometry, Volume 1, Theory, Marcel Dekker, New York, NY, 1969.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
´1
D3561 − 16 (2021)
an oxidant fuel flame. A monochromator isolates the charac- sufficiently high purity to permit its use without lessening the
teristic radiation from the hollow cathode lamp, and a photo- accuracy of the determination.
sensitive device measures the attenuated transmitted radiation,
8.2 Purity of Water—Unless otherwise indicated, reference
which may be read as absorbance units or directly as concen-
towatershallbeunderstoodtomeanreagentwaterconforming
tration on some instruments.
to Specification D1193, Type I. Other reagent water types may
4.2 Sincethevariableandsometimeshighconcentrationsof
be used provided it is first ascertained that the water is of
matrix materials in the waters and brines affect absorption
sufficiently high purity to permit its use without adversely
differently,itisdifficulttopreparestandardssufficientlysimilar
affecting the precision and bias of the test method. Type III
to the waters and brines. To overcome this difficulty, the
water was specified at the time of round robin testing of this
method of additions is used in which three identical samples
test method.
are prepared and varying amounts of a standard added to two
8.3 Lithium Solution, Standard (1 mL = 1 mg Li)—Dissolve
of them. The three samples are then aspirated, the concentra-
5.324goflithiumcarbonate(Li CO )inaminimumvolumeof
tion readings recorded, and the original sample concentration 2 3
HCl (1 + 1). Dilute to 1 L with water. One millilitre of this
calculated.
solution contains 1 mg of lithium. A purchased stock solution
5. Significance and Use of adequate purity is also acceptable.
5.1 Identification of a brackish water, seawater, or brine is
8.4 Potassium Solution, Stock (1 mL = 100 mg K)—
determined by comparison of the concentrations of their
Dissolve 190.7 g of potassium chloride (KCl) in water and
dissolved constituents. The results are used to evaluate the
dilute to 1 L with water. A purchased stock solution of
water as a possible pollutant, or as a commercial source of a
appropriate known purity is also acceptable.
valuable constituent such as lithium.
8.5 Potassium Solution, Standard (1 mL = 1 mg K)—
6. Interferences Dissolve 1.907 g of potassium chloride (KCl) in water and
dilute to 1 Lwith water. One millilitre of this solution contains
6.1 Ionization interference is controlled by adding large
1 mg of potassium. A purchased stock solution of appropriate
excesses of an easily ionized element. Sodium ion is added in
known purity is also acceptable.
the potassium and lithium determinations, and potassium ion is
added in the sodium determinations.
8.6 Sodium Solution, Stock (1 mL = 100 mg Na)—Dissolve
254.2 g of sodium chloride (NaCl) in water and dilute to 1 L
7. Apparatus
with water. A purchased stock solution of appropriate known
7.1 Atomic Absorption Spectrophotometer—The instrument
purity is also acceptable.
shall consist of an atomizer and burner, suitable pressure-
8.7 Sodium Solution, Standard (1 mL = 10 mg Na)—
regulating devices capable of maintaining constant oxidant and
Dissolve25.42gofsodiumchloride(NaCl)inwateranddilute
fuelpressureforthedurationofthetest,ahollowcathodelamp
to 1 L with water. One millilitre of this solution contains 1 mg
for each metal to be tested, an optical system capable of
of sodium. A purchased stock solution of appropriate known
isolating the desired line of radiation, an adjustable slit, a
purity is also acceptable.
photomultiplier tube or other photosensitive device as a light
measuring and amplifying device, and a readout mechanism
8.8 Oxidant:
for indicating the amount of absorbed radiation.
8.8.1 Air that has been cleaned and dried through a suitable
7.1.1 Multielement Hollow-Cathode Lamps.
filter to remove oil, water, and other foreign substances, is the
usual oxidant.
7.2 Pressure-Reducing Valves—The supplies of fuel and
oxidant shall be maintained at pressures somewhat higher than
8.9 Fuel:
the controlled operating pressure of the instrument by suitable
8.9.1 Acetylene—Standard, commercially available acety-
valves.
lene is the usual fuel. Acetone, always present in acetylene
cylinders, can be prevented from entering and damaging the
8. Reagents and Materials
burner head by replacing a cylinder that has only 689.4 kPa
8.1 Purity of Reagents—Reagent grade chemicals shall be
(100 psi) of acetylene remaining.
used in all tests. Unless otherwise indicated, it is intended that
allreagentsshallconformtothespecificationoftheCommittee 8.10 Filter Paper—Purchase suitable filter paper. Typically
on Analytical Reagents of the American Chemical Society, the filter papers have a pore size of 0.45-µm membrane.
where such specifications are available. Other grades may be Material such as fine-textured, acid-washed, ashless paper, or
used, provided it is first ascertained that the reagent is of glass fiber paper are acceptable. The user must first ascertain
that the filter paper is of sufficient purity to use without
adversely affecting the bias and precision of the test method.
ACS Reagent Chemicals, Specifications and Procedures for Reagents and
Standard-Grade Reference Materials, American Chemical Society, Washington,
9. Sampling
DC. For suggestions on the testing of reagents not listed by theAmerican Chemical
Society, see Analar Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset,
9.1 Collect the sample in accordance with the applicable
U.K., and the United States Pharmacopeia and National Formulary, U.S. Pharma-
copeial Convention, Inc. (USPC), Rockville, MD. ASTM standard (see Practices D3370).
´1
D3561 − 16 (2021)
10. Procedure making up to volume, add 0.5 mLof the potassium stock (8.4)
solution to the sodium standards and to a blank.Aspirate these
10.1 Potassium is determined at the 766.5-nm wavelength,
standards and the appropriate blank (for background setting)
lithiumatthe670.8-nmwavelength,andsodiumatthe330.2to
andadjustthecurvaturecontrols,ifnecessary,toobtainalinear
330.3-nm wavelength with an air-acetylene flame. For much
relationship between absorbance and the actual concentration
greater sensitivity, sodium is determined at the 589.0 to
of the standards.
589.6-nm wavelength.
10.3 Transfer an aliquot of water or brine (previously
10.2 Preliminary Calibration—Using micropipets prepare
filtered through a 0.45-µm filter [8.10]) to a 50-mL volumetric
lithium standards containing 1 to 5 mg/Lof lithium, potassium
standards containing 1 to 5 mg/L of potassium, and sodium flask. The specific gravity of the water or brine can be used to
estimate the lithium, potassium, or sodium content of the
standards containing 100 to 500 mg/L of sodium using the
standard lithium (8.3), potassium (8.5), and sodium (8.7) sample and, thereby, serve as a basis for selecting the aliquot
sizes that will contain about 0.05 mg of lithium, 0.05 mg of
solutions to 50-mL volumetric flasks. Before making up to
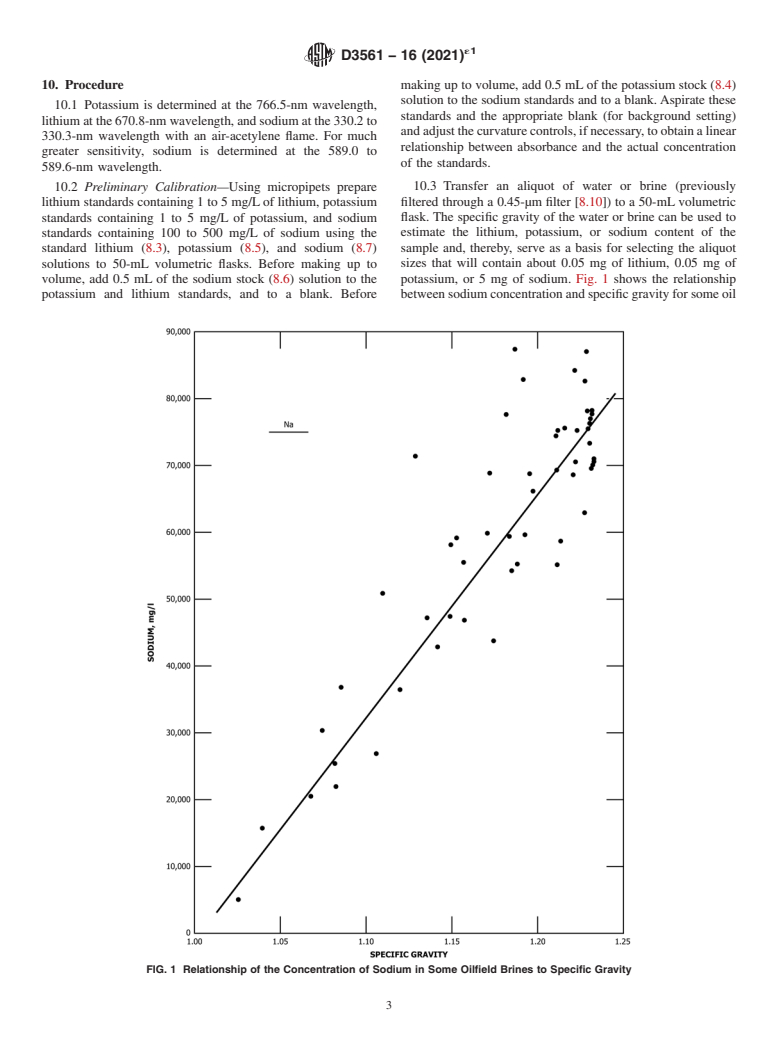
volume, add 0.5 mL of the sodium stock (8.6) solution to the potassium, or 5 mg of sodium. Fig. 1 shows the relationship
potassium and lithium standards, and to a blank. Before betweensodiumconcentrationandspecificgravityforsomeoil
FIG. 1 Relationship of the Concentration of Sodium in Some Oilfield Brines to Specific Gravity
´1
D3561 − 16 (2021)
field brines from the Smackover formation.The concentrations S 5 0.08905X1729
t
of sodium and also of lithium and potassium will not neces-
S 5 0.0295X1195
o
sarily correlate with the concentrations found in other forma-
where:
tions. Therefore, the user of this test method may find it
S = overall precision,
necessary to draw similar curves for brine samples taken from
t
S = single-operator precision, and
other formations. Add 0.5 mL of the sodium stock (8.6) o
X = concentration of lithium, potassium, or sodium
solution to the lithium and potassium samples and 0.5 mL of
determined, mg/L.
thepotassiumstock(8.4)solutiontothesodiumsamples,dilute
to volume, and aspirate. Calculate the approximate sample
12.2 The bias of this test method determined from recover-
concentration from the preliminary calibration readings, and
ies of known amounts of lithium, potassium, and sodium in a
determine the aliquot sizes that will contain about 0.05 mg of
series of prepared standards were as follows:
lithium, 0.05 mg of potassium, or 5 mg of sodium.
Lithium, Amount Added,
mg/L Recovery, % Relative
10.4 Transfer equal aliquots containing about 0.05 mg of
21.0 102.0
potassium or lithium, or 5 mg of sodium to three 50-mL
52.3 101.1
volumetric flasks.Add no potassium or lithium standard to the 74.1 100.5
164 95.0
first flask, using a micropipet add 0.05 mg to the second, and
Potassium, Amount Added,
0.1 mg to the third. For sodium, add no standard to the first
mg/L Recovery, % Relative
flask, 5 mg to the second, and 10 mg to the third. 591 111.0
1650 110.9
10.5 Add 0.5 mL of the sodium stock (8.6) solution to the
1670 113.2
1921 125.2
potassium and lithium samples and 0.5 mL of the potassium
Sodium, Amount Added,
stock (8.4) solution to the sodium samples, dilute to volume,
mg/L Recovery, % Relative
aspirate, and record the absorbance readings for each sample.
9 140 105.7
29 000 103.9
62 500 105.4
11. Calculation
66 200 108.3
11.1 Calculate the concentration of potassium, lithium, or
NOTE 1—The preceding precision and bias estimates are based on an
sodium ion in the original sample in milligrams per litre as
interlaboratory study of lithium, potassium, and sodium and interfering
follows: ionsasshowninTable1.Twoanalystsineachoffourlaboratoriesandone
analyst in each of two laboratories performed duplicate determinations on
11.2
each of two days. Practice D2777 was used in developing these precision
and bias estimates.
V A 3C
~ !
1 s std
Concentration, mg/L 5 (1)
V ~A 2 A !
12.3 It is the user’s responsibility to ensure the validity of
2 std s
this test method for waters of untested matrices.
where:
12.4 Precision and bias for this test method conforms to
V = volume of the dilute sample, mL,
Practice D2777 – 77, which was in place at the time of
V = volume of the original sample, mL,
collaborative testing. Under the allowances made in 1.
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.