ASTM E908-98(2004)
(Practice)Standard Practice for Calibrating Gaseous Reference Leaks
Standard Practice for Calibrating Gaseous Reference Leaks
ABSTRACT
This practice establishes the standard procedures for calibrating leak artifacts of a specified gas, that may be used for determining the response of leak detectors, or in other situations where a known small flow of gas is required. The purpose of this practice is to establish calibration without reference to other calibrated leaks in as straightforward a manner as possible using the likeliest available equipment. The two types of leaks considered here are Type I, which is pressure to vacuum, and Type II, which is pressure to atmosphere. Three calibration methods are described under each type of reference leak, as follows: Method A—accumulation comparison using a known volume of tracer gas at specified conditions of temperature and pressure as a reference; Method B—accumulation comparison using a reference leak artifact calibrated using Method A; and Method C—direct measurement of leak rate by timing the movement (displacement) of a liquid slug, by the leak, in a capillary tube of known dimensions.
SCOPE
1.1 This practice covers procedures for calibrating leak artifacts of a specified gas, that may be used for determining the response of leak detectors, or in other situations where a known small flow of gas is required. The purpose of this practice is to establish calibration without reference to other calibrated leaks in as straightforward a manner as possible using the likeliest available equipment. While the uncertainties associated with these procedures will most likely be greater than those obtained via traceable calibration chains (on the order of 10 %), these procedures allow independent means of establishing or verifying the leakage rate from leak artifacts of questionable history, or when traceable leak artifacts are not available.
1.2 Two types of leaks are considered:
1.2.1 Type I—Pressure to vacuum.
1.2.2 Type II—Pressure to atmosphere.
1.3 Three calibration methods are described under each type of reference leak:
1.3.1 Method A—Accumulation comparison, using a known volume of gas at specified conditions of temperature and pressure as a reference.
1.3.2 Method B—Accumulation comparison, using a leak artifact calibrated using Method A.
1.3.3 Method C—Displacement of a liquid slug, by the leak, in capillary tube of known dimensions.
1.4 The values stated in inch-pound units are to be regarded as the standard. The metric equivalents of inch-pound units may be appropriate.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E908 − 98(Reapproved 2004)
Standard Practice for
Calibrating Gaseous Reference Leaks
This standard is issued under the fixed designation E908; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This practice covers procedures for calibrating leak
E425Definitions of Terms Relating to Leak Testing (With-
artifacts of a specified gas, that may be used for determining
drawn 1991)
the response of leak detectors, or in other situations where a
E427PracticeforTestingforLeaksUsingtheHalogenLeak
known small flow of gas is required. The purpose of this
Detector(Alkali-Ion Diode)
practice is to establish calibration without reference to other
E479Guide for Preparation of a Leak Testing Specification
calibrated leaks in as straightforward a manner as possible
F134Test Methods for Determining Hermeticity of Electron
using the likeliest available equipment.While the uncertainties
Devices with a Helium Mass Spectrometer Leak Detector
associated with these procedures will most likely be greater
(Withdrawn 1996)
than those obtained via traceable calibration chains (on the
2.2 Other Documents:
order of 10%), these procedures allow independent means of
AVS 2.2-1968Method for Vacuum Leak Calibration
establishing or verifying the leakage rate from leak artifacts of
Recommended Practices for the Calibration and Use of
questionable history, or when traceable leak artifacts are not
Leaks
available.
1.2 Two types of leaks are considered:
3. Summary of Practice
1.2.1 Type I—Pressure to vacuum.
3.1 Method A—Accumulation comparison, using a known
1.2.2 Type II—Pressure to atmosphere.
volume of tracer gas:
3.1.1 This method uses a closed chamber of nonreactive
1.3 Threecalibrationmethodsaredescribedundereachtype
material having a means of removing all tracer gas and a
of reference leak:
connection to the tracer sensor.
1.3.1 MethodA—Accumulation comparison, using a known
3.1.2 A small, known quantity of tracer gas is discharged
volume of gas at specified conditions of temperature and
intothechamberandtheresponserecordedforaperiodoftime
pressure as a reference.
inwhichitisanticipatedtheunknownleakwillrequiretoreach
1.3.2 Method B—Accumulation comparison, using a leak
the same concentration.
artifact calibrated using Method A.
3.1.3 The tracer gas is removed from the chamber, and the
1.3.3 MethodC—Displacementofaliquidslug,bytheleak,
unknown leak is allowed to discharge into it until the sensor
in capillary tube of known dimensions.
response equals that of 3.1.2.
3.1.4 The leakage rate in mol/s can be calculated as:
1.4 The values stated in inch-pound units are to be regarded
as the standard. The metric equivalents of inch-pound units
Q 5PV ~t·R·T! (1)
m
may be appropriate.
where:
1.5 This standard does not purport to address all of the
P = pressure in known volume in atmospheres (1
safety concerns, if any, associated with its use. It is the
atm=101 325 Pa),
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
1 3
This practice is under the jurisdiction of ASTM Committee E07 on Nonde- The last approved version of this historical standard is referenced on
structive Testing and is the direct responsibility of Subcommittee E07.08 on Leak www.astm.org.
Testing Method. AvailablefromAVS,AmericanVacuumSociety,335E.45thStreet,NewYork,
Current edition approved May 1, 2004. Published June 2004. Originally N.Y., 10017.
approved in 1982. Last previous edition approved in 1998 as E908-98. DOI: C.D. Ehrlich and J.A. Basford, Journal of Vac. Sci, Technology, A(10), 1992,
10.1520/E0908-98R04. pp. 1–17.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E908 − 98 (2004)
NOTE 1—Other gases or detectors, or both, can be used with little
V = the volume of gas in cm introduced in 3.1.2,
difference in procedures or interferences.
t = the time in seconds required for the concentration in
4.1.2 PressureRise—Therewillinevitablybesomepressure
3.1.3 to equal that in 3.1.2,
R = gas constant=82.06=1 atm cm /mol/K, and
riseinaclosedevacuatedchamber,duetooutgassingandsmall
T = absolute temperature, K.
leaks.This may cause a decrease in ionization efficiency in the
spectrometer tube and thus a steadily declining signal as
3.1.5 It will be observed that chamber volume and sensor
indicated in Fig. 1. However, this effect should be quite
linearityarenotfactorsinthisequation.However,thechamber
constant from run to run, and so largely cancel out in final
volume must be selected to give a concentration within the
result.
sensorrange.Also,thisconcentrationmustalsobeachievedby
4.1.3 Helium Signal Rise—There will usually be a notice-
theunknownleakdischargingintothechamberinareasonable
ableincreaseinheliumsignalwhenthechamberisclosed,due
lengthoftimeandmustbeappropriatesoasnottosignificantly
to outgassing and in-leakage from the atmosphere as indicated
affect the equilibrium flow rate from the leak. This is particu-
in Fig. 1. Again, this will be a constant which mostly cancels
larly true of permeation leaks.
out.
3.2 MethodB—Accumulationcomparisonusingareference
4.1.4 SpectrometerSensitivityDrift—Thiswillbenoticedas
leak as calibrated in Method A, 3.1:
variations in zero and in reading levels with the same helium
3.2.1 This method is a means of extending the primary
input.Withproperlytunedandmaintainedsystemsoperatingat
calibration by a factor of up to 10, by comparing with
least one decade below maximum sensitivity, this should be a
previously-calibrated leak artifacts for longer periods of time.
minor effect.
−12
For example, a 5×10 mol/s leak that calibrated in Method
−13 4.1.5 Leaks—All detectable valve leaks and leaks from the
A at 300 s can be used for 30 s to calibrate a 5×10 mol/s
atmosphere should be repaired.
leak.
4.1.6 Barometric Variations—(Not applicable to sealed res-
3.2.2 When this method is used, it should be realized that
ervoir units.) If the gage used to measure the pressure in the
the total possible error will be at least doubled.
known volume is of the gage type, then account must be made
3.3 Method C—Direct measurement of leak rate by timing
of the local barometric pressure when calculating the absolute
the movement of a liquid slug in a capillary tube of known
pressure. This is probably true for falling pressures of the
dimensions:
known volume near 1 atmosphere or less.
3.3.1 The tube is closely coupled to the leak, and has a
4.1.7 Temperature Drift—Changes in temperature between
vent/fill valve to allow gas filling or positioning of the slug, or
measurements may result in slight variations in indicated
both, which is then driven by the leakage of the gas.
pressures. These should be recorded and compensated for
3.3.2 Due to capillary “friction,” this method is limited to a
accordingly.
−10 3
minimum leak size of about 4×10 mol/s (1 µPa·m /s).
4.2 Type I Leaks, atmosphere to vacuum, Method C:
4. Interferences
4.2.1 Liquid Slug Friction—This can be appreciable in
small capillaries. It should be measured and a correction made
4.1 Type I Leaks, atmosphere to vacuum, MethodsAand B:
for it.
4.1.1 Forthepurposesofthissection,itwillbeassumedthat
the gas is helium and the detector is the mass spectrometer 4.2.2 Vapor Pressure of Liquid—Water is the recommended
tuned for helium. liquid, and has a vapor pressure of about 20 mm Hg (3 kPa) at
FIG. 1 Typical Detector Curves and Deviation Limits
E908 − 98 (2004)
room temperature. This gives a theoretical increase in leak 5.1.2 Helium Supply with Pressure Regulator and Flowme-
3 −5 3
indication of 20/760 (3×10/1×10 ) or approximately 3%. ter (approximately 10 cm /s).
This correction should be added to the final result.
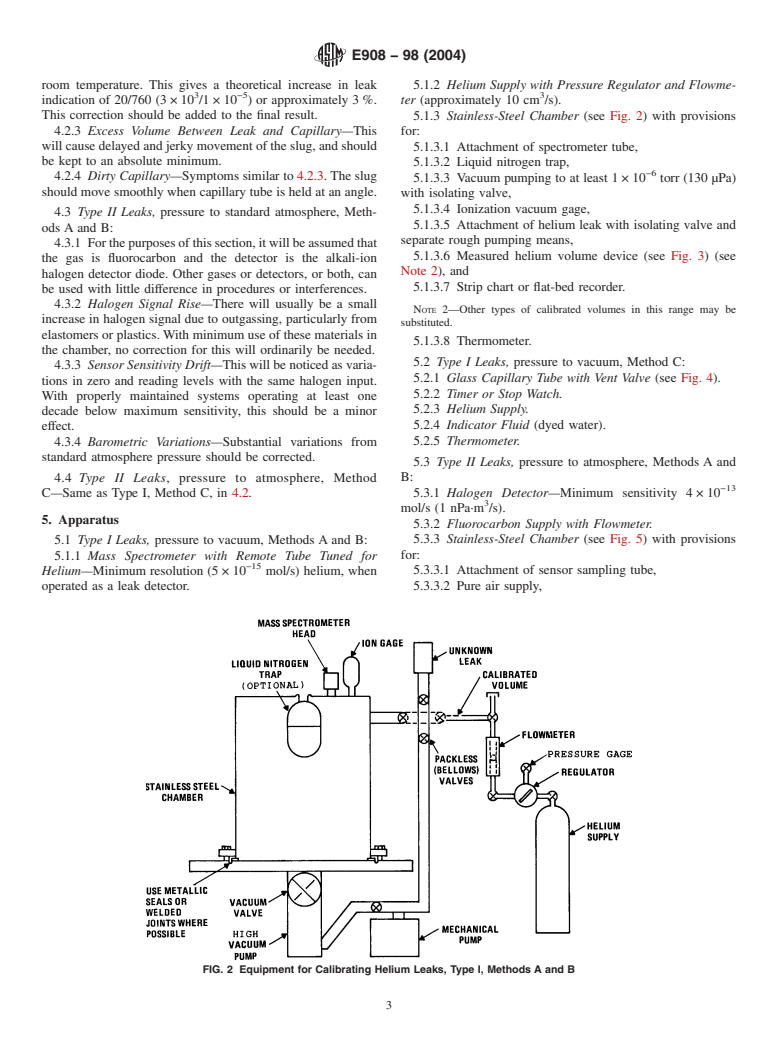
5.1.3 Stainless-Steel Chamber (see Fig. 2) with provisions
4.2.3 Excess Volume Between Leak and Capillary—This for:
willcausedelayedandjerkymovementoftheslug,andshould
5.1.3.1 Attachment of spectrometer tube,
be kept to an absolute minimum.
5.1.3.2 Liquid nitrogen trap,
−6
4.2.4 Dirty Capillary—Symptoms similar to 4.2.3.The slug
5.1.3.3 Vacuum pumping to at least 1×10 torr (130 µPa)
should move smoothly when capillary tube is held at an angle.
with isolating valve,
5.1.3.4 Ionization vacuum gage,
4.3 Type II Leaks, pressure to standard atmosphere, Meth-
5.1.3.5 Attachment of helium leak with isolating valve and
ods A and B:
separate rough pumping means,
4.3.1 Forthepurposesofthissection,itwillbeassumedthat
5.1.3.6 Measured helium volume device (see Fig. 3) (see
the gas is fluorocarbon and the detector is the alkali-ion
Note 2), and
halogen detector diode. Other gases or detectors, or both, can
5.1.3.7 Strip chart or flat-bed recorder.
be used with little difference in procedures or interferences.
4.3.2 Halogen Signal Rise—There will usually be a small
NOTE 2—Other types of calibrated volumes in this range may be
increase in halogen signal due to outgassing, particularly from
substituted.
elastomers or plastics.With minimum use of these materials in
5.1.3.8 Thermometer.
the chamber, no correction for this will ordinarily be needed.
5.2 Type I Leaks, pressure to vacuum, Method C:
4.3.3 SensorSensitivityDrift—Thiswillbenoticedasvaria-
5.2.1 Glass Capillary Tube with Vent Valve (see Fig. 4).
tions in zero and reading levels with the same halogen input.
5.2.2 Timer or Stop Watch.
With properly maintained systems operating at least one
5.2.3 Helium Supply.
decade below maximum sensitivity, this should be a minor
5.2.4 Indicator Fluid (dyed water).
effect.
4.3.4 Barometric Variations—Substantial variations from 5.2.5 Thermometer.
standard atmosphere pressure should be corrected.
5.3 Type II Leaks, pressure to atmosphere, Methods A and
4.4 Type II Leaks, pressure to atmosphere, Method B:
−13
C—Same as Type I, Method C, in 4.2. 5.3.1 Halogen Detector—Minimum sensitivity 4×10
mol/s (1 nPa·m /s).
5. Apparatus
5.3.2 Fluorocarbon Supply with Flowmeter.
5.1 Type I Leaks, pressure to vacuum, Methods A a
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.