ASTM D2650-99
(Test Method)Standard Test Method for Chemical Composition of Gases By Mass Spectrometry
Standard Test Method for Chemical Composition of Gases By Mass Spectrometry
SCOPE
1.1 This test method covers the quantitative analysis of gases containing specific combinations of the following components: hydrogen; hydrocarbons with up to six carbon atoms per molecule; carbon monoxide; carbon dioxide; mercaptans with one or two carbon atoms per molecule; hydrogen sulfide; and air (nitrogen, oxygen, and argon). This test method cannot be used for the determination of constituents present in amounts less than 0.1 mole%. Dimethylbutanes are assumed absent unless specifically sought. Note 1-Although experimental procedures described herein are uniform, calculation procedures vary with application. The following influences guide the selection of a particular calculation: qualitative mixture composition; minimum error due to components presumed absent; minimum cross interference between known components; maximum sensitivity to known components; low frequency and complexity of calibration; and type of computing machinery. Because of these influences, a tabulation of calculation procedures recommended for stated applications is presented in Section 12 (Table 1). Note 2-This test method was developed on Consolidated Electrodynamics Corporation Type 103 Mass Spectrometers. Users of other instruments may have to modify operating parameters and the calibration procedure.
1.2 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For a specific precautionary statement, see Note 6.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
An American National Standard
Designation:D2650–99
Standard Test Method for
Chemical Composition of Gases By Mass Spectrometry
This standard is issued under the fixed designation D 2650; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope D 1265 Practice for Sampling Liquefied Petroleum (LP)
Gases (Manual Method)
1.1 This test method covers the quantitative analysis of
D 1302 Method for Analysis of Carbureted Water Gas by
gases containing specific combinations of the following com-
the Mass Spectrometer
ponents: hydrogen; hydrocarbons with up to six carbon atoms
per molecule; carbon monoxide; carbon dioxide; mercaptans
3. Terminology
with one or two carbon atoms per molecule; hydrogen sulfide;
3.1 Definitions:
and air (nitrogen, oxygen, and argon). This test method cannot
3.1.1 mass number or m/e value of an ion—the quotient of
be used for the determination of constituents present in
the mass of that ion (given in atomic mass units) and its
amounts less than 0.1 mole %. Dimethylbutanes are assumed
positive charge (number of electrons lost during ionization).
absent unless specifically sought.
3.1.2 parent peak of a compound—the peak at which the
NOTE 1—Although experimental procedures described herein are uni-
m/e is equal to the sum of the atomic mass values for that
form, calculation procedures vary with application. The following influ-
compound. This peak is sometimes used as 100 % in comput-
ences guide the selection of a particular calculation: qualitative mixture
ing the cracking pattern coefficients.
composition; minimum error due to components presumed absent; mini-
3.1.3 base peak of a compound—the peak used as 100 % in
mum cross interference between known components; maximum sensitiv-
computing the cracking pattern coefficient.
ity to known components; low frequency and complexity of calibration;
and type of computing machinery. 3.1.4 cracking pattern coeffıcient—the ratio of a peak at any
Because of these influences, a tabulation of calculation procedures
m/e relative to its parent peak (or in some cases its base peak).
recommended for stated applications is presented in Section 12 (Table 1).
3.1.5 sensitivity—the height of any peak in the spectrum of
NOTE 2—This test method was developed on Consolidated Electrody-
the pure compound divided by the pressure prevailing in the
namics Corporation Type 103 Mass Spectrometers. Users of other
inlet system of the mass spectrometer immediately before
instruments may have to modify operating parameters and the calibration
opening the expansion bottle to leak.
procedure.
3.1.6 partial pressure—the pressure of any component in
1.2 This standard does not purport to address all of the
the inlet system before opening the expansion bottle to leak.
safety problems, if any, associated with its use. It is the
3.1.7 cracked gases—hydrocarbon gases that contain unsat-
responsibility of the user of this standard to establish appro-
urates.
priate safety and health practices and determine the applica-
3.1.8 straight-run gases—hydrocarbon gases that do not
bility of regulatory limitations prior to use. For a specific
contain unsaturates.
precautionary statement, see Note 5.
3.1.9 GLC—a gas-liquid chromatographic column that is
capable of separating the isomers of butenes, pentenes, hex-
2. Referenced Documents
anes, and hexenes.
2.1 ASTM Standards:
3.1.10 IR—infrared equipment capable of analyzing gases
D 1137 Method for Analysis of Natural Gases and Related
for the butene isomers.
Types of Gaseous Mixtures by the Mass Spectrometer
D 1145 Method of Sampling Natural Gas
4. Summary of Test Method
D 1247 Method of Sampling Manufactured Gas
4.1 The molecular species which make up a gaseous mix-
ture are dissociated and ionized by electron bombardment.The
positive ions of the different masses thus formed are acceler-
This test method is under the jurisdiction of ASTM Committee D-2 on
ated in an electrostatic field and separated in a magnetic field.
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
D02.04 on Hydrocarbon Analyses. The abundance of each mass present is recorded. The mixture
Current edition approved April 10, 1999. Published June 1999. Originally
published as D 2650 – 67 T. Last previous edition D 2650 –93.
2 4
Discontinued; see 1980 Annual Book of ASTM Standards, Part 26. Annual Book of ASTM Standards, Vol 05.01.
3 5
Discontinued; see 1986 Annual Book of ASTM Standards, Vol 05.05. Discontinued; see 1968 Annual Book of ASTM Standards, Part 19.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D2650–99
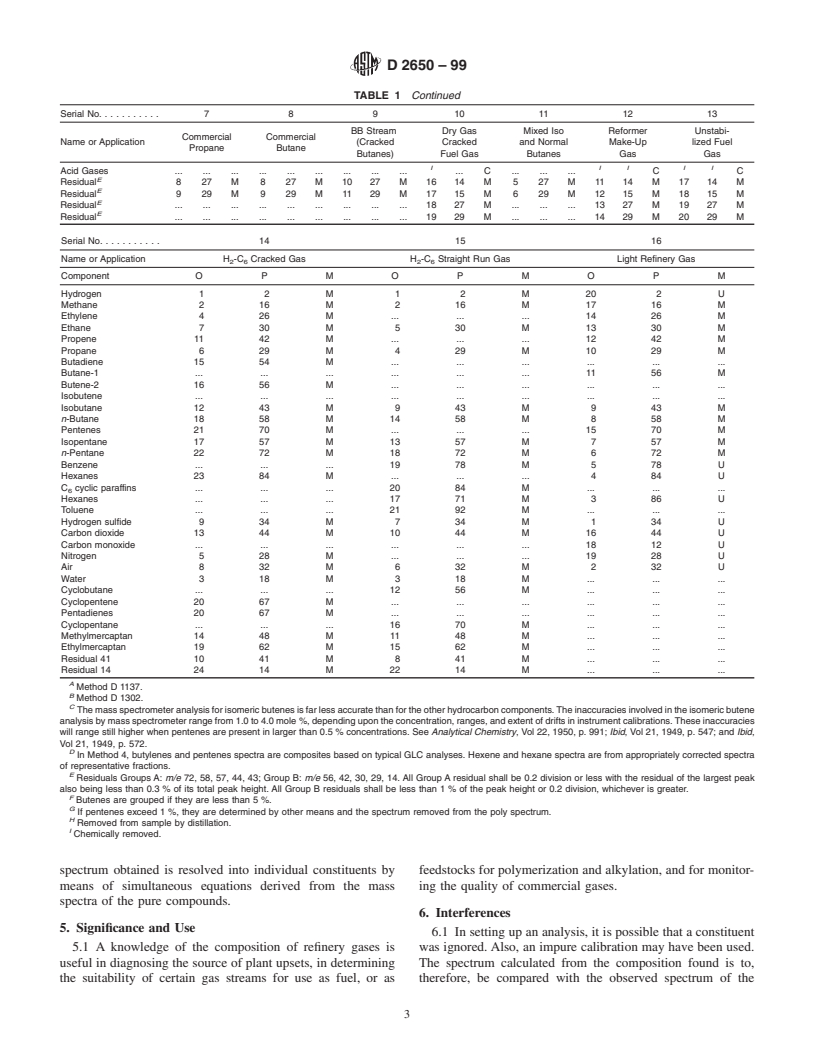
TABLE 1 Calculation Procedures for Mass Spectrometer Gas Analysis
NOTE 1—Coding of calculation procedures is as follows:
O = Order peaks are used in the calculation expressed serially from 1 to n, n being the total number of components.
P= m/e of peak used and prefix, M, if monoisotopic.
M = Method of computation
U = Unicomponent Peak Method
M = Simultaneous equations where “a” identifies the particular set of equations if more than one is used.
a
C = Chemically removed.
Residual = m/e of peak suitable as an independent check on the method.
SerialNo. 123456
B
D 1302
A
D 1137 Reformer
Name or Application Carbureted H -C C ,C iC
2 6 3 4 4
Natural Gas Gas
Water Gas
C C C C
Component O P M O P M O P MO P M O P MO P M
Hydrogen . . . 6 2 M 16 2 U 17 2 M 0 . . . . .
Methane 15 16 U 7 ⁄16 M 15 16 U 16 16 M 0 . . . . .
Ethylene 13 27 M2 12 27 M 13 26 U 15 26 M 0 . . . . .
Ethane 12 30 M2 8 30 M 12 30 U 13 30 M 0 . . . . .
Propene 10 42 M2 11 42 M 8 42 M2 12 42 M 6 42 M . . M
Propane 9 29 M2 9 29 M 3 44 M1 14 29 M 9 29 M 3 29 M
Butadiene . . . 9 . . 3 . . 10 54 M 9 . M . . M
Butene-1 8 56 M2 5 56 U 9 41 M2 8 56 M 8 41 M . . M
Butene-2 8 56 M2 5 56 U 10 55 M2 8 56 M 4 56 M . . M
Isobutene 8 56 M2 5 56 U 11 56 M2 8 56 M 5 39 M . . M
Isobutane 7 43 M2 5 . . 4 M43 M1 11 43 M 7 43 M 2 43 M
n-Butane 6 58 M2 4 58 U 5 58 M1 6 58 M 2 58 M 1 58 M
Pentenes . . . 3 70 U 2 70 U 9 55 M 3 70 M . . M
Isopentane . . . 3 . . 6 M57 M1 7 57 M 1 72 M . . .
n-Pentane 4 72 M2 2 72 U 7 72 M2 5 72 M . . . . . .
Benzene . . . 2 . . 7 . . 4 78 M . . . . . .
Hexanes . . . 2 . . 7 . . . . M . . . . . .
C cyclic paraffins . . . 2 . . 7 . . 3 84 M . . . . . .
Hexanes 5 57 M2 2 . . 1 71 U 2 86 M . . . . . .
Toluene . . . 2 . . 1 . . 1 92 M . . . . . .
Hydrogen sulfide 2 34 M1 2 . . 1 . . 21 34 M . . . . . .
Carbon dioxide 11 44 M2 10 44 M 1 . C 20 44 M . . . . . .
Carbon monoxide . . . 13 12 M 1 . C 18 28 M . . . . . .
Nitrogen 14 28 M2 14 14 M 14 28 U 19 14 M . . . . . .
Air 3 32 M1 1 32 U 14 . . 22 32 M 1 32 U . . .
DD
Helium 1 4 U 1 . . 14 . . . . . . . . .
SerialNo. 7 8 9 10 11 12 13
BB Stream Dry Gas Mixed Iso Reformer Unstabi-
Commercial Commercial
Name or Application (Cracked Cracked and Normal Make-Up lized Fuel
Propane Butane
Butanes) Fuel Gas Butanes Gas Gas
C C C C
Component O P M O P M O P MO P M O P MO P M O P M
Hydrogen . . . . . . . . . 15 2 M . . . 10 2 M 16 2 M
Methane . . . . . . . . . 14 16 M . . . 9 16 M 15 16 M
E
Ethylene 7 26 M . . . . . . 12 26 M . . . . . . 13 26 M
Ethane 6 30 M . . . . . . 11 30 M . . . 7 30 M 12 30 M
Propene 5 42 M 7 42 M 6 42 M 10 42 M . . . . . . 8 42 M
Propane 3 44 M 4 44 M 4 44 M 7 44 M 3 44 M 5 44 M 6 44 M
Butadiene . . . . . . 1 54 M 3 54 M . . . . . . 2 54 M
Butene-1 1 56 M 1 56 M 7 41 M 1 . . . . . . . . 9 41 M
Butene-2 1 56 M 1 56 M 8 56 M 1 56 M . . . . . . 10 56 M
FF FF F
Isobutene 1 M1 939 M 1 . 4 43 M . . . 11 39 M
Isobutane 4 43 M 5 43 M 5 43 M 8 43 M 1 58 M 6 43 M 7 43 M
n-Butane 2 58M 2 58M 2 58M 4 58M . . . 2 58M 3 58M
G
Pentenes . . . 6 70 M 70 U 9 70 M . . . 3 57 M . 70 U
Isopentane . . . 3 57 M 3 57 M 5 57 M 2 57 M 4 72 M 4 57 M
n-Pentane . . . . . . . . . 6 72 M . . . . . . 5 72 M
HH
Benzene . . . . . . . . . . . . . . . . . . D
HH
Hexanes . . . . . . . . . . . . . . . . . . D
HH
C cyclic paraffins . . . . . . . . . . . . . . . . . . D
HH
Hexanes . . . . . . . . . . . . . . . . . . D
HH
Toluene . . . . . . . . . . . . . . . . . . D
I II II
Hydrogen sulfide . . . . . . . . . . C . . . C C
I II II
Carbon dioxide . . . . . . . . . . C . . . C C
Carbon monoxide . . . . . . . . . 13 28 M . . . 8 28 M 14 28 M
Nitrogen . . . . . . . . . . . . . . . . . . . . .
Air . . . . . . . . . 2 32 M . . . 1 32 M 1 32 M
D2650–99
TABLE 1 Continued
SerialNo. 7 8 9 10 11 12 13
BB Stream Dry Gas Mixed Iso Reformer Unstabi-
Commercial Commercial
Name or Application (Cracked Cracked and Normal Make-Up lized Fuel
Propane Butane
Butanes) Fuel Gas Butanes Gas Gas
I II II
Acid Gases . . . . . . . . . . C . . . C C
E
Residual 8 27M 8 27M10 27 M16 14M 5 27M 11 14 M17 14M
E
Residual 9 29M 9 29M 11 29 M17 15M 6 29M12 15 M18 15M
E
Residual . . . . . . . . . 18 27 M . . . 13 27 M 19 27 M
E
Residual . . . . . . . . . 19 29 M . . . 14 29 M 20 29 M
SerialNo. 14 15 16
Name or Application H -C Cracked Gas H -C Straight Run Gas Light Refinery Gas
2 6 2 6
Component O P M O P M O P M
Hydrogen 1 2 M 1 2 M 20 2 U
Methane 2 16 M 2 16 M 17 16 M
Ethylene 4 26 M . . . 14 26 M
Ethane 7 30 M 5 30 M 13 30 M
Propene 11 42 M . . . 12 42 M
Propane 6 29 M 4 29 M 10 29 M
Butadiene 15 54 M . . . . . .
Butane-1 . . . . . . 11 56 M
Butene-2 16 56 M . . . . . .
Isobutene . . . . . . . . .
Isobutane 12 43 M 9 43 M 9 43 M
n-Butane 18 58 M 14 58 M 8 58 M
Pentenes 21 70 M . . . 15 70 M
Isopentane 17 57 M 13 57 M 7 57 M
n-Pentane 22 72 M 18 72 M 6 72 M
Benzene . . . 19 78 M 5 78 U
Hexanes 23 84 M . . . 4 84 U
C cyclic paraffins . . . 20 84 M . . .
Hexanes . . . 17 71 M 3 86 U
Toluene . . . 21 92 M . . .
Hydrogen sulfide 9 34 M 7 34 M 1 34 U
Carbon dioxide 13 44 M 10 44 M 16 44 U
Carbon monoxide . . . . . . 18 12 U
Nitrogen 5 28 M . . . 19 28 U
Air 8 32 M 6 32 M 2 32 U
Water 3 18 M 3 18 M . . .
Cyclobutane . . . 12 56 M . . .
Cyclopentene 20 67 M . . . . . .
Pentadienes 20 67 M . . . . . .
Cyclopentane . . . 16 70 M . . .
Methylmercaptan 14 48 M 11 48 M . . .
Ethylmercaptan 19 62 M 15 62 M . . .
Residual 41 10 41 M 8 41 M . . .
Residual 14 24 14 M 22 14 M . . .
A
Method D 1137.
B
Method D 1302.
C
Themassspectrometeranalysisforisomericbutenesisfarlessaccuratethanfortheotherhydrocarboncomponents.Theinaccuraciesinvolvedintheisomericbutene
analysisbymassspectrometerrangefrom1.0to4.0mole %,dependingupontheconcentration,ranges,andextentofdriftsininstrumentcalibrations.Theseinaccuracies
will range still higher when pentenes are present in larger than 0.5 % concentrations. See Analytical Chemistry, Vol 22, 1950, p. 991; Ibid, Vol 21, 1949, p. 547; and Ibid,
Vol 21, 1949, p. 572.
D
In Method 4, butylenes and pentenes spectra are composites based on typical GLC analyses. Hexene and hexane spectra are from appropriately corrected spectra
of representative fractions.
E
Residuals Groups A: m/e 72, 58, 57, 44, 43; Group B: m/e 56, 42, 30, 29, 14. All Group A residual shall be 0.2 division or less with the residual of the largest peak
also being less than 0.3 % of its total peak height. All Group B residuals shall be less than 1 % of the peak height or 0.2 division, whichever is greater.
F
Butenes are grouped if they are less than 5 %.
G
If pentenes exceed 1 %, they are determined by other means and the spectrum removed from the poly spectrum.
H
Removed from sample by distillation.
I
Chemically removed.
spectrum obtained is resolved into individual constituents by feedstocks for polymerization and alkylation, and for monitor-
means of simultaneous equations derived from the mass ing the quality of commercial gases.
spectra of the pure compounds.
6. Interferences
5. Significance and Use
6.1 In setting up an analysis, it is possible that a constituent
5.1 A knowledge of the composition of refinery gases is was ignored. Also, an impure calibration may have been used.
useful in diagnosing the source of plant upsets, in determining The spectrum calculated from the composition found is to,
the suitability of certain gas streams for use as fuel, or as therefore, be compared with the observed spectrum of the
D2650–99
mixture at masses independent of the original calculation.
3 hydrogen
4 n-butane
Differences so computed, called residuals, should as a general
5 hydrogen
rule be less than 1 % of the original mixture peak for an
acceptable analysis. Masses suitable for this calculation are 10.1.2 If the 43/58 and 43/29 ratios of the first two runs do
tabulated with each calculation procedure. not agree with 0.8 %, further runs must be made until agree-
ment is attained, either by adjusting the temperature of the
NOTE 3—Another strategy employed to reduce interferences and in-
ionization chamber or by other techniques commonly used by
crease accuracy consists of using spectra which have been corrected for
the laboratory. In any case, the three 43/58 and 43/29 ratios
contributions caused by the rare isotopes of carbon and hydrogen.
must agree within 0.8 % and the three butane sensitivities
7. Apparatus
within 1 %. The two hydrogen sensitivities must agree within
1.5 %.Astandard gas sample can also be used as an additional
7.1 Mass Spectrometer—Any mass spectrometer can be
check.
used with this test method that shall be proven by performance
10.2 Reference Stand
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.