ASTM F1717-96
(Test Method)Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model
Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model
SCOPE
1.1 These test methods cover the materials and methods for the static and fatigue testing of spinal implant assemblies in a vertebrectomy model. The test materials for most combinations of spinal implant components can be specific depending on the intended spinal location and intended method of application to the spine.
1.2 These test methods are intended to provide a basis for the mechanical comparison among past, present, and future spinal implant assemblies. They allow comparison of spinal implant constructs with different intended spinal locations and methods of application to the spine. These test methods are not intended to define levels of performance, since sufficient knowledge is not available to predict the consequences of the use of a particular device.
1.3 These test methods set out guidelines for load types and methods of applying loads. Methods for three static load types and one fatigue test are defined for the comparative evaluation of spinal implant assemblies.
1.4 These test methods establish guidelines for measuring displacements, determining the yield load, and evaluating the stiffness and strength of the spinal implant assembly.
1.5 Some spinal constructs may not be testable in all test configurations.
1.6 Values stated in SI units are to be regarded as standard.
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 1717 – 96
Standard Test Methods for
Static and Fatigue for Spinal Implant Constructs in a
Corpectomy Model
This standard is issued under the fixed designation F 1717; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope ized Stress-Life (S-N) and Strain-Life (e-N) Fatigue Data
E 1150 Definitions of Terms Relating to Fatigue
1.1 This standard covers the materials and methods for the
F 1582 Terminology Relating to Spinal Implants
static and fatigue testing of spinal implant assemblies in a
corpectomy model. The test materials for most combinations of
3. Terminology
spinal implant components can be specific depending on the
3.1 Definitions:
intended spinal location and intended method of application to
3.1.1 For definitions of terms relating to the test method, see
the spine.
Terminology E 6, Terminology F 1582 and Definitions E 1150.
1.2 This standard is intended to provide a basis for the
3.2 Definitions of Terms Specific to This Standard:
mechanical comparison among past, present and future spinal
3.2.1 active length of the longitudinal element—the straight
implant assemblies. It allows comparison of spinal implant
line distance between the center of attachment of the superior
constructs with different intended spinal locations and methods
anchor and the center of attachment of the inferior anchor.
of application to the spine. These test methods are not intended
3.2.2 angular displacement at 2 % offset yield (degrees)—
to define levels of performance, since sufficient knowledge is
the angular displacement of a construct measured via the
not available to predict the consequences of the use of a
actuator that produces a permanent angular displacement in the
particular device.
X-Y plane equal to 0.020 times the torsional aspect ratio (see
1.3 The standard sets out guidelines for load types and
Point A in Fig. 1).
methods of applying loads. Methods for three static load types
3.2.3 block moment arm—the perpendicular to the applied
and one fatigue test are defined for the comparative evaluation
load between the insertion point of an anchor and the axis of
of spinal implant assemblies.
the hidge pin.
1.4 This standard establishes guidelines for measuring dis-
3.2.4 compressive or tensile bending stiffness (N/mm)—the
placements, determining the yield load, evaluating the stiffness
compressive or tensile bending yield force divided by elastic
and strength of the spinal implant assembly.
displacement (see the initial slope of line BC in Fig. 1).
1.5 Some spinal constructs may not be testable in all test
3.2.5 compressive or tensile bending ultimate load (N)—the
configurations.
maximum compressive or tensile force in X-Z plane applied to
1.6 Values stated in SI units are to be regarded as standard.
a spinal implant assembly (see the force at Point E in Fig. 1).
1.7 This standard does not purport to address all of the
The ultimate load should be a function of the device and not of
safety concerns, if any, associated with its use. It is the
the load cell or testing machine.
responsibility of the user of this standard to establish appro-
3.2.6 compressive or tensile bending yield load (N)—the
priate safety and health practices and determine the applica-
compressive or tensile bending force in X-Z plane necessary to
bility of regulatory limitations prior to use.
produce a permanent deformation equal to 0.020 times the
2. Referenced Documents active length of the longitudinal element (see the force at Point
D in Fig. 1).
2.1 ASTM Standards:
3.2.7 coordinate system/axes—three orthogonal axes are
D 638 Test Method for Tensile Properties of Plastic
3 defined in Fig. 2 and Fig. 3. The anterior-posterior axis is X
E 4 Practices for Force Verification of Testing Machines
with positive being anterior. The medial-lateral axis is Y with
E 6 Terminology Relating to Methods of Mechanical Test-
3 left being positive when viewed posteriorly. The superior-
ing
inferior axis is Z with superior being positive.
E 739 Practice for Statistical Analysis of Linear or Linear-
3.2.8 displacement at 2 % offset yield (mm)—the displace-
ment of a construct measured via the actuator that produces a
These test methods are under the jurisdiction of ASTM Committee F-4 on
permanent deformation equal to 0.020 times the active length
Medical and Surgical Materials and Devices and are the direct responsibility of
of the longitudinal element (see Point A in Fig. 1).
Subcommittee F04.25on Spinal Devices.
Current edition approved June 10, 1996. Published December 1996.
Annual Book of ASTM Standards, Vol 08.01.
3 4
Annual Book of ASTM Standards, Vol 03.01. Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
F 1717
FIG. 2 A Standard Bilateral Construct Containing Screw, Rod and
Screw
FIG. 1 Typical Load Displacement Curve or Torque Angulation
Curve
3.2.9 elastic angular displacement (degrees)—the angular
displacement at 2 % offset yield (see Point A in Fig. 1) minus
the 2 % offset angular displacement (see Point B in Fig. 1).
(The distance between Point A and Point B in Fig. 1.)
3.2.10 elastic displacement (mm)—the displacement at 2 %
offset yield (see Point A in Fig. 1) minus the 2 % offset
displacement (see Point B in Fig. 1). (The distance between
Point A and Point B in Fig. 1.)
3.2.11 failure—permanent deformation resulting from frac-
ture, plastic deformation or loosening beyond the ultimate
displacement or loosening that renders the spinal implant
assembly ineffective or unable to adequately resist load.
3.2.12 fatigue life—the number of loading cycle, N,ofa
specified character that the spinal implant assembly sustains
before failure of a specified nature occurs (see Definitions
E 1150).
3.2.13 insertion point of an anchor—the location where the
anchor is attached to the test block. The insertion points shown
in Figs. 2-15 are to be adhered to if possible. In situations
FIG. 3 A Bilateral Hook, Rod, Screw, and Transverse Element
where the design of the spinal implant assembly or the
Construct
manufacturer’s surgical instructions for installation dictate
otherwise, the attachment points may deviate from these
dimensions. spine intended for the application of the spinal implant
3.2.14 intended method of application—spinal implant as- assembly. Spinal implant assemblies are developed for specific
semblies contain different types of anchors. Each type of spinal locations such as the anterior cervical spine or the
anchor has an intended method of application to the spine. posterior thoracolumbar, lumbar and lumbosacral spine.
3.2.15 intended spinal location—the anatomic region of the 3.2.16 hinge pin—the cylindrical rod connecting a test
F 1717
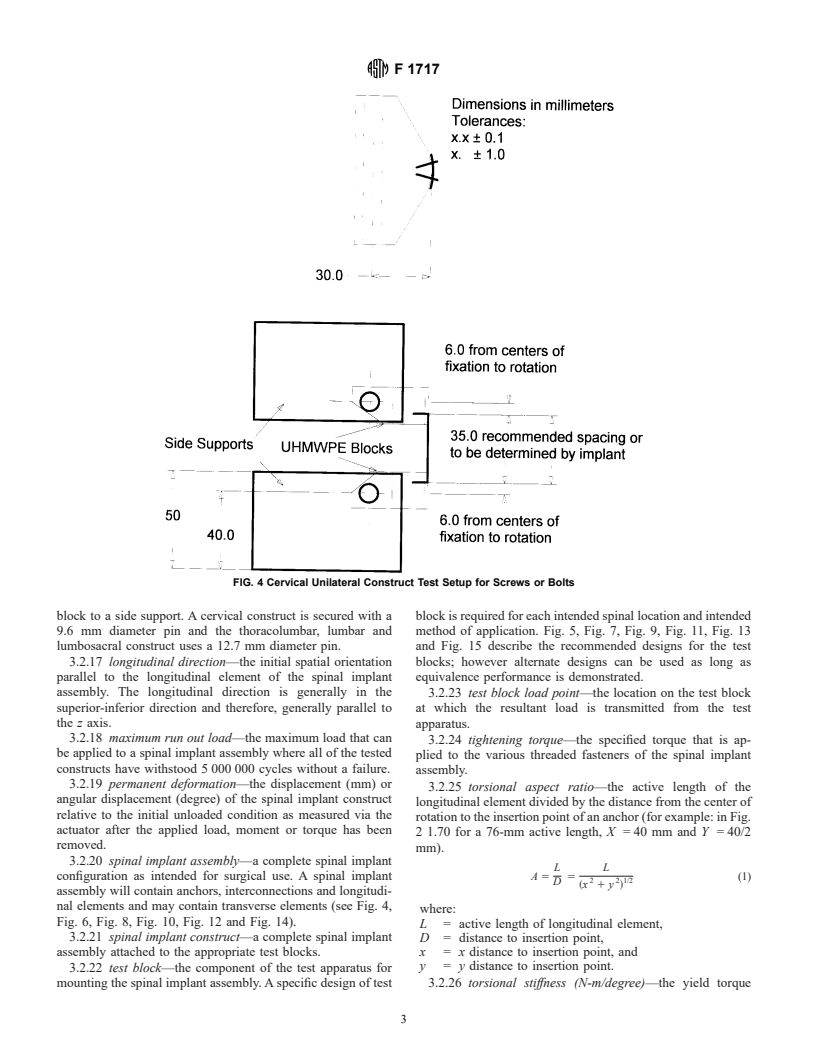
FIG. 4 Cervical Unilateral Construct Test Setup for Screws or Bolts
block to a side support. A cervical construct is secured with a block is required for each intended spinal location and intended
9.6 mm diameter pin and the thoracolumbar, lumbar and method of application. Fig. 5, Fig. 7, Fig. 9, Fig. 11, Fig. 13
lumbosacral construct uses a 12.7 mm diameter pin. and Fig. 15 describe the recommended designs for the test
3.2.17 longitudinal direction—the initial spatial orientation blocks; however alternate designs can be used as long as
parallel to the longitudinal element of the spinal implant equivalence performance is demonstrated.
assembly. The longitudinal direction is generally in the 3.2.23 test block load point—the location on the test block
superior-inferior direction and therefore, generally parallel to
at which the resultant load is transmitted from the test
the z axis. apparatus.
3.2.18 maximum run out load—the maximum load that can
3.2.24 tightening torque—the specified torque that is ap-
be applied to a spinal implant assembly where all of the tested
plied to the various threaded fasteners of the spinal implant
constructs have withstood 5 000 000 cycles without a failure.
assembly.
3.2.19 permanent deformation—the displacement (mm) or
3.2.25 torsional aspect ratio—the active length of the
angular displacement (degree) of the spinal implant construct
longitudinal element divided by the distance from the center of
relative to the initial unloaded condition as measured via the
rotation to the insertion point of an anchor (for example: in Fig.
actuator after the applied load, moment or torque has been
2 1.70 for a 76-mm active length, X =40mmand Y = 40/2
removed.
mm).
3.2.20 spinal implant assembly—a complete spinal implant
L L
configuration as intended for surgical use. A spinal implant A 5 5 (1)
2 2 1/2
D
x 1 y !
~
assembly will contain anchors, interconnections and longitudi-
nal elements and may contain transverse elements (see Fig. 4,
where:
Fig. 6, Fig. 8, Fig. 10, Fig. 12 and Fig. 14).
L = active length of longitudinal element,
3.2.21 spinal implant construct—a complete spinal implant
D = distance to insertion point,
assembly attached to the appropriate test blocks. x = x distance to insertion point, and
y = y distance to insertion point.
3.2.22 test block—the component of the test apparatus for
mounting the spinal implant assembly. A specific design of test 3.2.26 torsional stiffness (N-m/degree)—the yield torque
F 1717
FIG. 5 Cervical Unilateral UHWMPE Block for Screws or Bolts
(N-m) divided by elastic angular displacement (degrees) (the 3.2.30 ultimate displacement (mm)—the displacement asso-
initial slope of line BC in Fig. 1). ciated with the ultimate load, ultimate bending load or ultimate
3.2.27 torsional ultimate load (N-m)—the maximum torque torque (the displacement at Point F in Fig. 1).
in X-Y plane applied to a spinal implant assembly (the torque 3.2.31 yield torque (N-m)—the torque in X-Y plane required
at Point E in Fig. 1). The ultimate torque should be a function to produce a permanent displacement of 0.020 times the
of the device and not of the load cell or testing machine. torsional aspect ratio (the torque at Point D in Fig. 1).
3.2.28 two percent (2 %) offset angular displacement 3.2.32 zero displacement intercept (mm)—the intersection
(degrees)—a permanent angular displacement in the X-Y plane of the straight line section of the load displacement curve and
measured via the actuator equal to 0.020 times the torsional the zero load axis (the zero displacement reference Point 0 in
aspect ratio (for example: 1.95° for 1.70 3 0.02 3 180°/pi) Fig. 1).
(see Point B in Fig. 1).
4. Summary of Test Methods
3.2.29 two percent (2 %) offset displacement (mm)—a per-
manent deformation measured via the actuator equal to 0.020 4.1 Similar test methods are proposed for the mechanical
times the active length of the longitudinal element (for ex- evaluation of cervical spinal implant assemblies (see Fig. 4,
ample: 1.52 mm for a 76 mm active length of the longitudinal Fig. 6 and Fig. 8) and thoracolumbar, lumbar and lumbosacral
element or 0.70 mm for 35 mm) (see Point B in Fig. 1). spinal implant assemblies (see Fig. 10, Fig. 12 and Fig. 14).
F 1717
FIG. 6 Cervical Bilateral Construct Test Setup for Screws or Bolts
4.2 Testing of the spinal implant assemblies will simulate a 4.5 The intended method of application of the spinal im-
corpectomy model via a large gap between two Ultra High plant assembly may vary for specific anatomic regions and
Molecular Weight Polyethylene (UHMWPE) test blocks. The clinical indications. Spinal implant assemblies contain different
UHMWPE used to manufacture the test blocks should have a types of anchors. Each type of anchor has an intended method
tensile breaking strength equal to 40 6 3 MPa (see Specifica- of application to the spine. For example, one assembly may
tion F 638). The UHMWPE test blocks (see Fig. 5, Fig. 7, Fig. include anterior vertebral body screws and rods (see Fig. 2),
9, Fig. 11, Fig. 13, and Fig. 15) will eliminate the effects of the while another assembly may contain posterior sacral screws,
variability of bone properties and morphometry. Alternate hooks, rods and transverse elements (see Fig. 3). The block
designs of test blocks may be used as long as equivalence moment arm of a test configuration will be independent of the
performance is demonstrated. intended method of application of a spinal implant assembly,
4.3 Three static mechanical tests and one dynamic test will therefore the test data for different intended method of appli-
evaluate the spinal implant assemblies. The three static me- cation may be compared.
chanical tests are compression bending, tensile bending and
5. Significance and Use
torsion. The dynamic test is a compression bending fatigue.
4.4 A specific clinical indication generally requires a spe- 5.1 Spinal implants are generally composed of several
cific spinal implant assembly. Spinal implant assemblies will components which, when connected together, form a spinal
be evaluated with test configurations which simulate the implant assembly. Spinal implant assemblies are designed to
clinical requirements for the intended spinal location. The provide some stability to the spine while arthrodesis takes
intended spinal locations are both anterior (see Fig. 4) and place. This standard outlines standard materials and methods
posterior (see Fig. 6 and Fig. 8) surfaces of the cervical spine for the evaluation of different spinal implant assemblies so that
or both anterior (see Fig. 10) and posterior (see Fig. 12 and Fig. comparison between different designs may be facilitated.
14) surfaces of the thoracolumbar, lumbar and lumbosacral 5.2 This standard is used to quantify the static and dynamic
spine. The block moment arm (see 6.6) for a test configuration mechanical characteristics of different designs of spinal im-
depends on the intended spinal location. The cervical spine plant assemblies. The mechanical tests are conducted in vitro
configuration (see Fig. 5, Fig. 7, and Fig. 9) specifies one block using simplified load schemes and do not attempt to mimic the
moment arm, while a larger block moment arm (see Fig. 11, complex loads of the spine.
Fig. 13, and Fig. 15) is specified for the thoracolumbar, lumbar 5.3 The loads applied to the spinal implant assemblies in
and lumbosacral spine. vivo will, in general, differ from the loading configurations
F 1717
FIG. 7 Cervical Bilateral UHMWPE Block for Screws or Bolts
used in this standard. The results obta
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.