ASTM G170-06(2020)e1
(Guide)Standard Guide for Evaluating and Qualifying Oilfield and Refinery Corrosion Inhibitors in the Laboratory
Standard Guide for Evaluating and Qualifying Oilfield and Refinery Corrosion Inhibitors in the Laboratory
SIGNIFICANCE AND USE
5.1 Corrosion inhibitors continue to play a key role in controlling internal corrosion associated with oil and gas production and transportation. This results primarily from the industry’s extensive use of carbon and low alloy steels, which, for many applications, are economic materials of construction that generally exhibit poor corrosion resistance. As a consequence, there is a strong reliance on inhibitor deployment for achieving cost-effective corrosion control, especially for treating long flowlines and main export pipelines (1).5
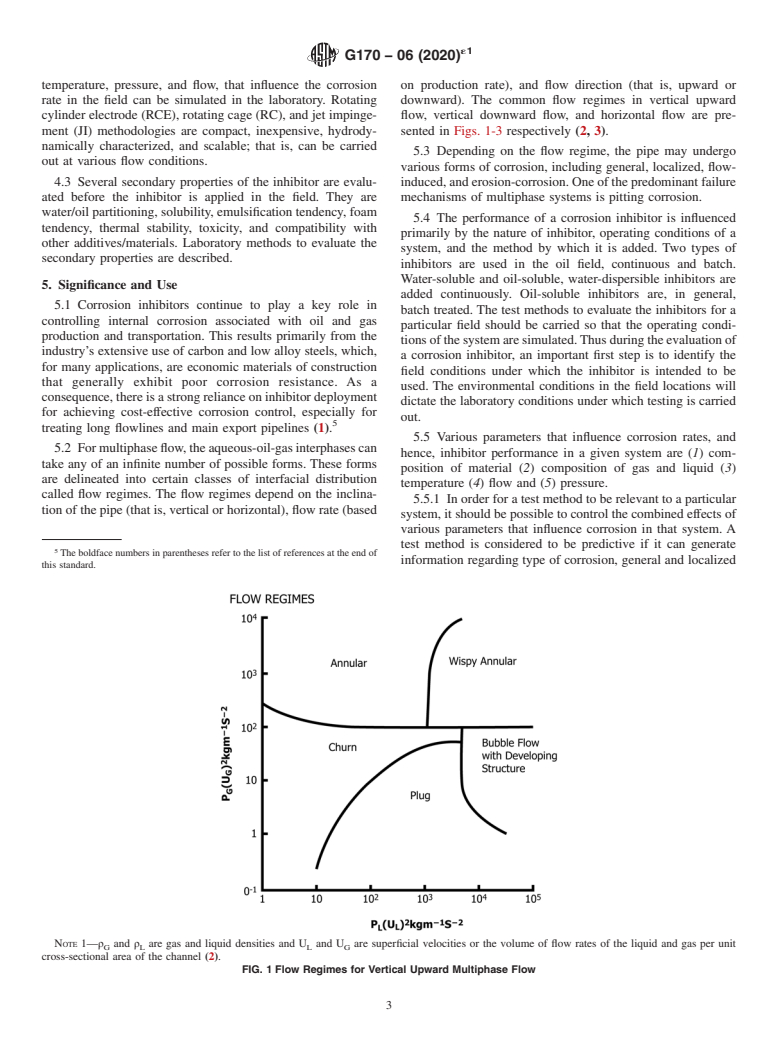
5.2 For multiphase flow, the aqueous-oil-gas interphases can take any of an infinite number of possible forms. These forms are delineated into certain classes of interfacial distribution called flow regimes. The flow regimes depend on the inclination of the pipe (that is, vertical or horizontal), flow rate (based on production rate), and flow direction (that is, upward or downward). The common flow regimes in vertical upward flow, vertical downward flow, and horizontal flow are presented in Figs. 1-3 respectively (2, 3).
5.14 To develop an inhibitor selection strategy, in addition to inhibitor efficiency, several other key performance factors need to be evaluated: (1) water/oil partitioning, (2) solubility, (3) emulsification tendency, (4) foaming tendency, (5) thermal stability, (6) toxicity, and (7) compatibility with other additives/materials.
SCOPE
1.1 This guide covers some generally accepted laboratory methodologies that are used for evaluating corrosion inhibitors for oilfield and refinery applications in well defined flow conditions.
1.2 This guide does not cover detailed calculations and methods, but rather covers a range of approaches which have found application in inhibitor evaluation.
1.3 Only those methodologies that have found wide acceptance in inhibitor evaluation are considered in this guide.
1.4 This guide is intended to assist in the selection of methodologies that can be used for evaluating corrosion inhibitors.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory requirements prior to use.
1.7 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
´1
Designation:G170 −06 (Reapproved 2020)
Standard Guide for
Evaluating and Qualifying Oilfield and Refinery Corrosion
Inhibitors in the Laboratory
This standard is issued under the fixed designation G170; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
ε NOTE—Units statement was inserted in 1.5, and Terminology G15 was replaced by Terminology G193 editorially, and
editorial changes were made throughout in December 2020.
1. Scope 2. Referenced Documents
1.1 This guide covers some generally accepted laboratory
2.1 ASTM Standards:
methodologies that are used for evaluating corrosion inhibitors
D1141 Practice for the Preparation of Substitute Ocean
for oilfield and refinery applications in well defined flow
Water
conditions.
D4410 Terminology for Fluvial Sediment
1.2 This guide does not cover detailed calculations and
G1 Practice for Preparing, Cleaning, and Evaluating Corro-
methods, but rather covers a range of approaches which have
sion Test Specimens
found application in inhibitor evaluation.
G3 Practice for Conventions Applicable to Electrochemical
Measurements in Corrosion Testing
1.3 Only those methodologies that have found wide accep-
G5 Reference Test Method for Making Potentiodynamic
tance in inhibitor evaluation are considered in this guide.
Anodic Polarization Measurements
1.4 This guide is intended to assist in the selection of
G16 Guide for Applying Statistics to Analysis of Corrosion
methodologies that can be used for evaluating corrosion
Data
inhibitors.
G31 Guide for Laboratory Immersion Corrosion Testing of
1.5 The values stated in SI units are to be regarded as
Metals
standard. No other units of measurement are included in this
G46 Guide for Examination and Evaluation of Pitting Cor-
standard.
rosion
1.6 This standard does not purport to address all of the
G59 Test Method for Conducting Potentiodynamic Polariza-
safety concerns, if any, associated with its use. It is the
tion Resistance Measurements
responsibility of the user of this standard to establish appro-
G96 Guide for Online Monitoring of Corrosion in Plant
priate safety, health, and environmental practices and deter-
Equipment (Electrical and Electrochemical Methods)
mine the applicability of regulatory requirements prior to use.
G102 Practice for Calculation of Corrosion Rates and Re-
1.7 This international standard was developed in accor-
lated Information from Electrochemical Measurements
dance with internationally recognized principles on standard-
G106 Practice for Verification of Algorithm and Equipment
ization established in the Decision on Principles for the
for Electrochemical Impedance Measurements
Development of International Standards, Guides and Recom-
G111 Guide for Corrosion Tests in High Temperature or
mendations issued by the World Trade Organization Technical
High Pressure Environment, or Both
Barriers to Trade (TBT) Committee.
G193 Terminology and Acronyms Relating to Corrosion
This guide is under the jurisdiction ofASTM Committee G01 on Corrosion of
Metals and is the direct responsibility of Subcommittee G01.05 on Laboratory
Corrosion Tests. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved Nov. 1, 2020. Published December 2020. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 2001. Last previous edition approved in 2012 as G170 – 06 (2012). Standards volume information, refer to the standard’s Document Summary page on
DOI: 10.1520/G0170-06R20E01. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
´1
G170−06 (2020)
2.2 NACE Standards: 3.1.9 gas to oil ratio (GOR)—ratio of the amount of gas and
NACE-5A195 State-of-the-Art Report on Controlled-Flow oil transported through a pipe over a given time.
Laboratory Corrosion Test, Houston, TX, NACE Interna-
3.1.10 high-pressure—a pressure above ambient atmo-
tional Publication, Item No. 24187, December 1995
spheric pressure that cannot be contained in normal laboratory
NACE-ID196 Laboratory Test Methods for Evaluating Oil-
glassware. Typically, this is greater than 0.07 MPa (10 psig).
Field Corrosion Inhibitors, Houston, TX, NACE Interna-
3.1.11 high-temperature—temperatures above ambient
tional Publication, Item No. 24192, December 1996
laboratory temperature where sustained heating of the environ-
NACE-TM0196 Standard Test Method “Chemical Resis-
ment is required.
tance of Polymeric Materials by Periodic Evaluation,”
3.1.12 laboratory methodology—a small laboratory experi-
Houston, TX, NACE International Publication, Item No.
mental set up, that is used to generate the corrosion. Examples
21226, 1996
oflaboratorymethodologiesincluderotatingcylinderelectrode
2.3 ISO Standards:
(RCE), rotating cage (RC), and jet impingement (JI) under
ISO 696 Surface Active Agents — Measurements of Foam-
flowing conditions.
ing Power Modified Ross-Miles Method
ISO 6614 Petroleum Products — Determination of Water 3.1.13 live water—aqueous solution obtained from a pipe-
Separability of Petroleum Oils and Synthetic Fluids line or well. Usually live water is protected from atmospheric
oxygen.
3. Terminology
3.1.14 mass transfer coeffıcient (k, m/s)—the rate at which
3.1 Definitions of Terms Specific to This Standard:
the reactants (or products) are transferred to the surface (or
3.1.1 atmosphericpressureexperiment—anexperimentcon-
removed from the surface).
ducted at the ambient atmospheric pressure (typically less than
3.1.15 measuring technique—technique for determining the
0.07 MPa (10 psig)), using normal laboratory glassware.
rate of corrosion and the inhibitor efficiency. Examples of
3.1.2 batch inhibitor—an inhibitor that forms a film on the
measuring techniques are mass loss, linear polarization resis-
metal surface that persists to effect inhibition.
tance (LPR), electrochemical impedance spectroscopy (EIS),
3.1.3 batch treatment—a method of applying a batch inhibi- electrical resistance (ER), and potentiodynamic polarization
(PP) methods.
tor. Batch inhibitors are applied as a plug between pigs or as
slugs of chemical poured down the well bore. The batch
3.1.16 multiphase flow—simultaneous passage or transport
inhibitor is dissolved or dispersed in a medium, usually
of more than one phase, where the phases have different states
hydrocarbon and the inhibited solution is allowed to be in
(gas, liquid, and solid) or the same state (liquid), but different
contact with the surface that is to be protected for a fixed
fluid characteristics (viscosity, density, and specific gravity).
amountoftime.Duringthisperiod,theinhibitorfilmisformed
3.1.17 synthetic water—a synthetic solution prepared in the
on the surface and protects the surface during the passage of
laboratory using various chemicals. The composition is based
multiphase flow, for example, oil/water/gas.
on the composition of fluid found in an oil production system.
3.1.4 continuous inhibitor—an inhibitor that is continuously
3.1.18 Schmidt Number (Sc)—a measure of the ratio of the
injected into the system in order to effect inhibition. Since the
hydrodynamic boundary layer to the diffusion boundary layer.
surface receives full exposure to the inhibitor, the film repair is
This dimensionless parameter is equal to kinematic viscosity
continuous.
divided by diffusion coefficient.
3.1.5 emulsification-tendency—a property of an inhibitor
3.1.19 wall shear stress (τ, N/m )—a force per unit area on
that causes the water and hydrocarbon mixture to form an
the pipe due to fluid friction.
emulsion.Theemulsionformedcanbequitedifficulttoremove
3.2 The terminology used herein, if not specifically defined
and this will lead to separation difficulties in the production
otherwise, shall be in accordance with Terminology D4410 or
facilities.
G193. Definitions provided herein and not given in Terminol-
3.1.6 film persistency—ability of inhibitor film (usually
ogy D4410 or G193 are limited only to this guide.
batchinhibitor)towithstandtheforces(forexample,flow)that
tend to destroy the film over time.
4. Summary of Guide
3.1.7 flow loop—an experimental pipe that contains various
4.1 Inhibitor evaluation in the laboratory consists of two
corrosionprobestomonitorcorrosionrates.Aflowloopcanbe
steps(1)evaluationofinhibitorefficiencyand(2)evaluationof
constructed in the laboratory or attached to an operating
secondary inhibitor properties.
system.
4.2 Four laboratory methodologies, flow loop, rotating cyl-
3.1.8 foaming tendency—tendency of inhibitor in solution
inder electrode (RCE), rotating cage (RC), and jet impinge-
(water or hydrocarbon) to create and stabilize foam when gas
ment(JI)areavailabletoevaluatetheinhibitorefficiencyinthe
is purged through the solution.
laboratory. All four methodologies can be operated at atmo-
spheric and high pressure conditions. The corrosion rates can
AvailablefromNACEInternational(NACE),15835ParkTenPl.,Houston,TX
be measured using mass loss or electrochemical methods.
77084, http://www.nace.org.
Using the methodologies, several variables, compositions of
Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
4th Floor, New York, NY 10036, http://www.ansi.org. material, composition of environment (gas and liquid),
´1
G170−06 (2020)
temperature, pressure, and flow, that influence the corrosion on production rate), and flow direction (that is, upward or
rate in the field can be simulated in the laboratory. Rotating downward). The common flow regimes in vertical upward
cylinder electrode (RCE), rotating cage (RC), and jet impinge- flow, vertical downward flow, and horizontal flow are pre-
ment (JI) methodologies are compact, inexpensive, hydrody- sented in Figs. 1-3 respectively (2, 3).
namically characterized, and scalable; that is, can be carried
5.3 Depending on the flow regime, the pipe may undergo
out at various flow conditions.
various forms of corrosion, including general, localized, flow-
4.3 Several secondary properties of the inhibitor are evalu- induced,anderosion-corrosion.Oneofthepredominantfailure
ated before the inhibitor is applied in the field. They are mechanisms of multiphase systems is pitting corrosion.
water/oil partitioning, solubility, emulsification tendency, foam
5.4 The performance of a corrosion inhibitor is influenced
tendency, thermal stability, toxicity, and compatibility with
primarily by the nature of inhibitor, operating conditions of a
other additives/materials. Laboratory methods to evaluate the
system, and the method by which it is added. Two types of
secondary properties are described.
inhibitors are used in the oil field, continuous and batch.
Water-soluble and oil-soluble, water-dispersible inhibitors are
5. Significance and Use
added continuously. Oil-soluble inhibitors are, in general,
5.1 Corrosion inhibitors continue to play a key role in
batch treated. The test methods to evaluate the inhibitors for a
controlling internal corrosion associated with oil and gas
particular field should be carried so that the operating condi-
production and transportation. This results primarily from the
tionsofthesystemaresimulated.Thusduringtheevaluationof
industry’s extensive use of carbon and low alloy steels, which,
a corrosion inhibitor, an important first step is to identify the
for many applications, are economic materials of construction
field conditions under which the inhibitor is intended to be
that generally exhibit poor corrosion resistance. As a
used. The environmental conditions in the field locations will
consequence,thereisastrongrelianceoninhibitordeployment
dictate the laboratory conditions under which testing is carried
for achieving cost-effective corrosion control, especially for
out.
treating long flowlines and main export pipelines (1).
5.5 Various parameters that influence corrosion rates, and
5.2 Formultiphaseflow,theaqueous-oil-gasinterphasescan
hence, inhibitor performance in a given system are (1) com-
take any of an infinite number of possible forms. These forms
position of material (2) composition of gas and liquid (3)
are delineated into certain classes of interfacial distribution
temperature (4) flow and (5) pressure.
called flow regimes. The flow regimes depend on the inclina-
5.5.1 In order for a test method to be relevant to a particular
tion of the pipe (that is, vertical or horizontal), flow rate (based
system, it should be possible to control the combined effects of
various parameters that influence corrosion in that system. A
test method is considered to be predictive if it can generate
The boldface numbers in parentheses refer to the list of references at the end of
information regarding type of corrosion, general and localized
this standard.
NOTE 1—ρ and ρ are gas and liquid densities and U and U are superficial velocities or the volume of flow rates of the liquid and gas per unit
G L L G
cross-sectional area of the channel (2).
FIG. 1Flow Regimes for Vertical Upward Multiphase Flow
´1
G170−06 (2020)
5.8 Flow is an indirect variable, and simulation of flow in
the laboratory is not direct. For this reason, the hydrodynamic
flow parameters are determined, and then the laboratory
corrosion tests are conducted under the calculated hydrody-
namic parameters. The fundamental assumption in this ap-
proach is that, when the hydrodynamic parameters of different
geometries are the same, then the corrosion mechanism will be
the same. Under these conditions, the corrosion rate and the
efficiency of corrosion inhibition in the laboratory and in the
field are similar. The commonly used hydrodynamic param-
eters are wall shear stress, Reynolds number, and mass transfer
coefficient (3, 5).
5.9 Neither the flow rate (m/s) nor dimensionless param-
eterscanbedirectlyrelatedtothelocalhydrodynamicforcesat
the material surface that may be responsible for accelerated
localized attack. Local hydrodynamic forces are influenced by
severalfactorsincludingpipeinclination,position(thatis,3,6,
9 o’clock), presence of bends, deposits, edges, welds,
FIG. 2Flow Regimes for Vertical Downward Flow (2)
expansion, and contraction. The flow rate and dimensionless
parameters describe only bulk, or average, properties of the
dynamic system. Thus the wall shear stress and mass transfer
corrosion rates, nature
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.