ASTM D4266-96(2009)e1
(Test Method)Standard Test Methods for Precoat Capacity of Powdered Ion-Exchange Resins
Standard Test Methods for Precoat Capacity of Powdered Ion-Exchange Resins
SIGNIFICANCE AND USE
The salt removal capacity of a powdered resin precoat is limited by the capacity of either the anion-exchange resin or the cation-exchange resin contained in it. Applications include condensate polishing in fossil-fueled electric generating plants, as well as condensate polishing, spent fuel pool water treatment, reactor water treatment, and low-level radioactive liquid waste treatment in nuclear-powered electric generating plants.
By determining the ion-exchange capacity profile of either a cation exchange resin or an anion-exchange resin (capacity expended per unit of time under specific conditions), it is possible to estimate runlength and remaining capacity when treating a liquid of the same makeup. Although they cannot accurately predict performance during condenser leaks, these test methods are useful for determining operating capacities as measured under the test conditions used.
These test methods may be used to monitor the performance of either powdered anion-exchange resin or powdered cation-exchange resin. The total capacity of either resin depends primarily upon the number density of ion-exchange sites within the resin. The operating capacity is a function of the total capacity, degree of conversion to the desired ionic form when received, and properties of the resin and the system that affect ion exchange kinetics.
SCOPE
1.1 These test methods cover the determination of the operating ion-exchange capacity of both powdered cation-exchange resins (hydrogen form) and powdered anion-exchange resins (hydroxide form). These test methods are intended for use in testing new powdered ion-exchange resins when used for the treatment of water. The following two test methods are included:
Sections Test Method A—Operating Capacity, Anion-Exchange
Resin, Hydroxide Form7 to 15 Test Method B—Operating Capacity, Cation-Exchange
Resin, Hydrogen Form16 to 24
1.2 The values stated in SI units are to be regarded as the standard. The inch-pound units given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
´1
Designation: D4266 − 96 (Reapproved 2009)
Standard Test Methods for
Precoat Capacity of Powdered Ion-Exchange Resins
This standard is issued under the fixed designation D4266; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
ε NOTE—Updated 5.2 editorially in June 2009.
1. Scope E200Practice for Preparation, Standardization, and Storage
of Standard and Reagent Solutions for ChemicalAnalysis
1.1 These test methods cover the determination of the
operating ion-exchange capacity of both powdered cation-
3. Terminology
exchange resins (hydrogen form) and powdered anion-
exchange resins (hydroxide form). These test methods are 3.1 Definitions of Terms Specific to This Standard:
intended for use in testing new powdered ion-exchange resins
3.1.1 For definitions of other terms used in these test
when used for the treatment of water. The following two test
methods, refer to Terminology D1129.
methods are included:
3.1.2 powdered ion-exchange material, n—an ion-exchange
resin that has undergone post-manufacturing size reduction to
Sections
Test Method A—Operating Capacity, Anion-Exchange 7 to 15
less than 300 µm.
Resin, Hydroxide Form
Test Method B—Operating Capacity, Cation-Exchange 16 to 24
3.1.3 resin dosage, n—theweightofmixedresinappliedper
Resin, Hydrogen Form
unit area of precoatable filter surface. This is expressed as dry
1.2 The values stated in SI units are to be regarded as the pounds per square foot.
standard. The inch-pound units given in parentheses are for
3.1.4 resin floc, n—thatvoluminousaggregateformedwhen
information only.
powdered anion-exchange resin and powdered cation-
1.3 This standard does not purport to address all of the
exchange resin are slurried together in an aqueous suspension.
safety concerns, if any, associated with its use. It is the
3.1.5 resin ratio, n—the ratio of the weights of powdered
responsibility of the user of this standard to establish appro-
cation-exchange resin to powdered anion-exchange resin used
priate safety and health practices and determine the applica-
to prepare a resin slurry. If not otherwise indicated, it is
bility of regulatory limitations prior to use.
understood to be the ratio of the dry resin weights.
2. Referenced Documents
4. Significance and Use
2.1 ASTM Standards:
4.1 Thesaltremovalcapacityofapowderedresinprecoatis
D1125Test Methods for Electrical Conductivity and Resis-
limited by the capacity of either the anion-exchange resin or
tivity of Water
the cation-exchange resin contained in it.Applications include
D1129Terminology Relating to Water
condensatepolishinginfossil-fueledelectricgeneratingplants,
D1193Specification for Reagent Water
as well as condensate polishing, spent fuel pool water
D2687PracticesforSamplingParticulateIon-ExchangeMa-
treatment, reactor water treatment, and low-level radioactive
terials
liquid waste treatment in nuclear-powered electric generating
D4456Test Methods for Physical and Chemical Properties
plants.
of Powdered Ion Exchange Resins
4.2 By determining the ion-exchange capacity profile of
either a cation exchange resin or an anion-exchange resin
(capacity expended per unit of time under specific conditions),
These test methods are under the jurisdiction of ASTM Committee D19 on
it is possible to estimate runlength and remaining capacity
Water and are the direct responsibility of Subcommittee D19.08 on Membranes and
Ion Exchange Materials.
when treating a liquid of the same makeup. Although they
Current edition approved May 1, 2009. Published June 2009. Originally
cannot accurately predict performance during condenser leaks,
approved in 1983. Last previous edition approved in 2007 as D4266–96 (2007).
thesetestmethodsareusefulfordeterminingoperatingcapaci-
DOI: 10.1520/D4266-96R09E01.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or ties as measured under the test conditions used.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
4.3 These test methods may be used to monitor the perfor-
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. mance of either powdered anion-exchange resin or powdered
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
´1
D4266 − 96 (2009)
cation-exchange resin. The total capacity of either resin de- 9.1.1 Water Pump—adjustable between 0 to 7.57 L/min (0
5 4
pendsprimarilyuponthenumberdensityofion-exchangesites to 2 gal/min) at 2.76×10 Pa (40 psig) pressure.
within the resin. The operating capacity is a function of the 9.1.2 Pressure Gauges (two), 0 to 4.137×10 Pa (0 to 60
total capacity, degree of conversion to the desired ionic form psig) with appropriate snubbers.
when received, and properties of the resin and the system that 9.1.3 Disk Filter Holder, 142-mm diameter with sufficient
affect ion exchange kinetics. clearance above the filter disk to allow for uniform application
of resin precoat.
5. Purity of Reagents 9.1.4 Filter-Disk, 142-mm diameter, with nominal retention
rating of 25 to 30 µm and absolute retention rating of 40 to
5.1 Reagent grade chemicals shall be used in all tests.
60µm.
Unlessotherwiseindicated,itisintendedthatallreagentsshall
9.1.5 Flow Meter, 0 to 1.89 L/min (0 to 30 gal/h) with
conform to the specifications of the Committee on Analytical
regulating valve.
Reagents of the American Chemical Society, where such
9.1.6 Beaker, stainless steel, 4 L to volume with bulkhead
specifications are available.
fittings installed at tubing penetrations.
5.2 Purity of Water—Unless otherwise indicated, references
9.1.7 Chemical Pump, with pumping rate between
−6 −5
to water shall be understood to mean Type II reagent water,
8.33×10 and 8.33×10 L/s (30 to 300 mL/h) at 3.45×10
Specification D1193.
6 Pa (500 psig) pressure. Suction tubing should be 3.2-mm
( ⁄8-in.) outside diameter stainless steel and discharge tubing
6. Sampling
should be 1.6-mm ( ⁄16-in.) outside diameter stainless steel.
6.1 Obtain a representative sample of the powdered ion-
9.2 Electrical Conductivity Measurement Apparatus, con-
exchange resin in accordance with Practices D2687 but sub-
forming to the requirements given in Test Methods D1125,
stituting a 12.5-mm ( ⁄2-in.) inside diameter tube.
Method B.
TEST METHOD A—OPERATING CAPACITY,
10. Reagents
ANION-EXCHANGE RESIN, HYDROXIDE FORM
10.1 Hydrochloric Acid Solution, Standard (0.10 N)—
Prepare and standardize as described in Practice E200.
7. Scope
10.2 Polyacrylic Acid Solution, Standard (1+99)—Pipet 1
7.1 This test method covers the determination of ion-
mL of polyacrylic acid (25 weight% solids, MW<50 000)
exchange capacity, on a dry weight basis, of new powdered
into a 100 mL volumetric flask and dilute to 100 mL with
anion-exchange resins in the hydroxide form.
water. Mix well. Prepare this solution fresh daily.
7.2 The ion-exchange capacity obtainable in commercial
installations depends not only upon the initial state of the 11. Sample Preparation
powdered resin, but also on how the resin floc is prepared and
11.1 Selection of Proper Sample Weight—Use a resin dos-
2 2
applied, on the condition of the equipment on which it is to be
age of 1 kg/m (0.2 lb/ft ) and a resin ratio of 2+1.
used, and the pH and general chemistry of the water system
being treated. Thus, this test method has comparative rather
than predictive value and provides an upper limit on exchange
The sole source of supply of the Millipore pump (ZPN100400) apparatus
knowntothecommitteeatthistimeistheMilliporeCorporation,290ConcordRd.,
capacity that may be expected.
Billerica, MA 01821. If you are aware of alternative suppliers, please provide this
information to ASTM International Headquarters. Your comments will receive
8. Summary of Test Method
careful consideration at a meeting of the responsible technical committee, which
you may attend.
8.1 The powdered anion-exchange resin to be tested is
The sole source of supply of the Millipore filter holder (YY2214230) with
slurried with an appropriate amount of powdered cation-
acryliccylinder(XX4214201)andaccessoriesapparatusknowntothecommitteeat
this time is the Millipore Corporation, 290 Concord Rd., Billerica, MA 01821. If
exchange resin in the hydrogen form, and the resulting floc is
you are aware of alternative suppliers, please provide this information to ASTM
precoatedontoafilterdisk.Thenadilutestandardizedsolution
International Headquarters. Your comments will receive careful consideration at a
of a strong acid is fed to the precoat while monitoring the
meeting of the responsible technical committee, which you may attend.
The sole source of supply of the BG or DG filter apparatus known to the
effluent stream conductometrically.
committee at this time is the Pall Corporation, 30 Sea Cliff Ave., Glen Cove, NY
11542. If you are aware of alternative suppliers, please provide this information to
9. Apparatus
ASTM International Headquarters. Your comments will receive careful consider-
ation at a meeting of the responsible technical committee, which you may attend.
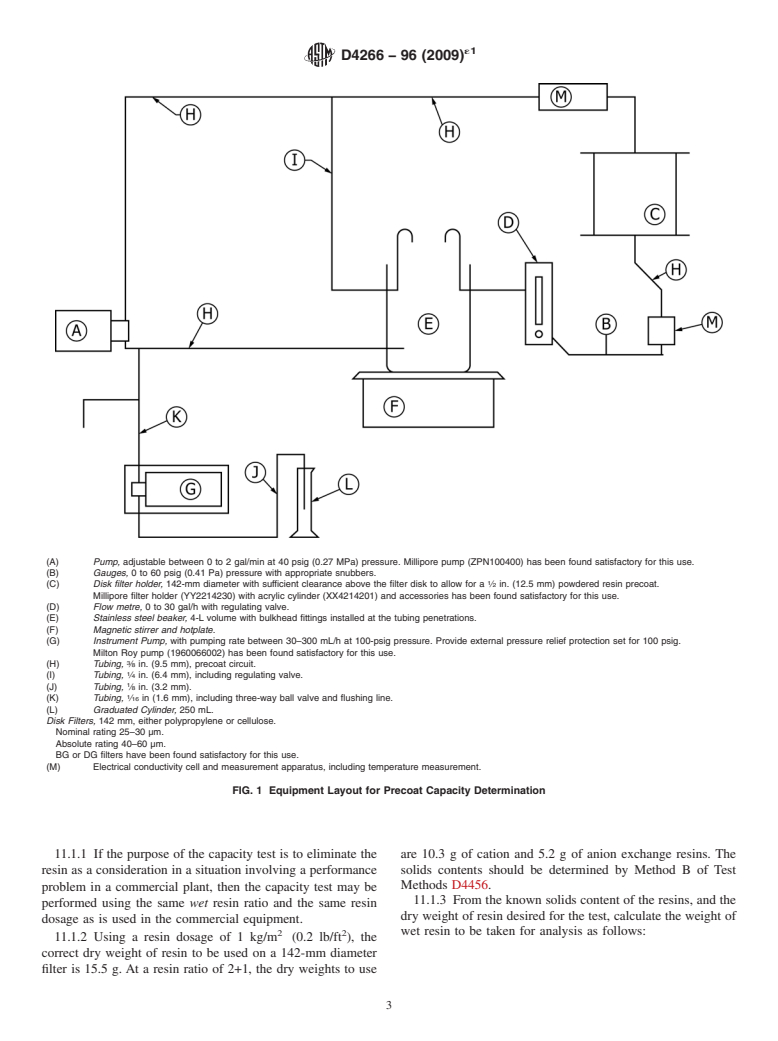
9.1 Test apparatus, as shown in Fig. 1, with the following
The sole source of supply of the Milton Roy pump (1960066002) apparatus
components:
known to the committee at this time is Milton Roy USA, 201 Ivyland Rd., Ivyland,
PA18974. If you are aware of alternative suppliers, please provide this information
to ASTM International Headquarters. Your comments will receive careful consid-
erationatameetingoftheresponsibletechnicalcommittee, whichyoumayattend.
3 8
Reagent Chemicals, American Chemical Society Specifications, American The sole source of supply of the Accumer (1510) apparatus known to the
Chemical Society, Washington, DC. For suggestions on the testing of reagents not committee at this time is the Rohm and Haas Company, 100 Independence Mall
listed by the American Chemical Society, see Analar Standards for Laboratory West, Philadelphia, PA 19106. If you are aware of alternative suppliers, please
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia provide this information toASTM International Headquarters.Your comments will
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville, receive careful consideration at a meeting of the responsible technical committee,
MD. which you may attend.
´1
D4266 − 96 (2009)
(A) Pump, adjustable between 0 to 2 gal/min at 40 psig (0.27 MPa) pressure. Millipore pump (ZPN10
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.