ASTM G82-98(2014)
(Guide)Standard Guide for Development and Use of a Galvanic Series for Predicting Galvanic Corrosion Performance
Standard Guide for Development and Use of a Galvanic Series for Predicting Galvanic Corrosion Performance
SIGNIFICANCE AND USE
4.1 When two dissimilar metals in electrical contact are exposed to a common electrolyte, one of the metals can undergo increased corrosion while the other can show decreased corrosion. This type of accelerated corrosion is referred to as galvanic corrosion. Because galvanic corrosion can occur at a high rate, it is important that a means be available to alert the user of products or equipment that involve the use of dissimilar metal combinations in an electrolyte of the possible effects of galvanic corrosion.
4.2 One method that is used to predict the effects of galvanic corrosion is to develop a galvanic series by arranging a list of the materials of interest in order of observed corrosion potentials in the environment and conditions of interest. The metal that will suffer increased corrosion in a galvanic couple in that environment can then be predicted from the relative position of the two metals in the series.
4.3 Types of Galvanic Series:
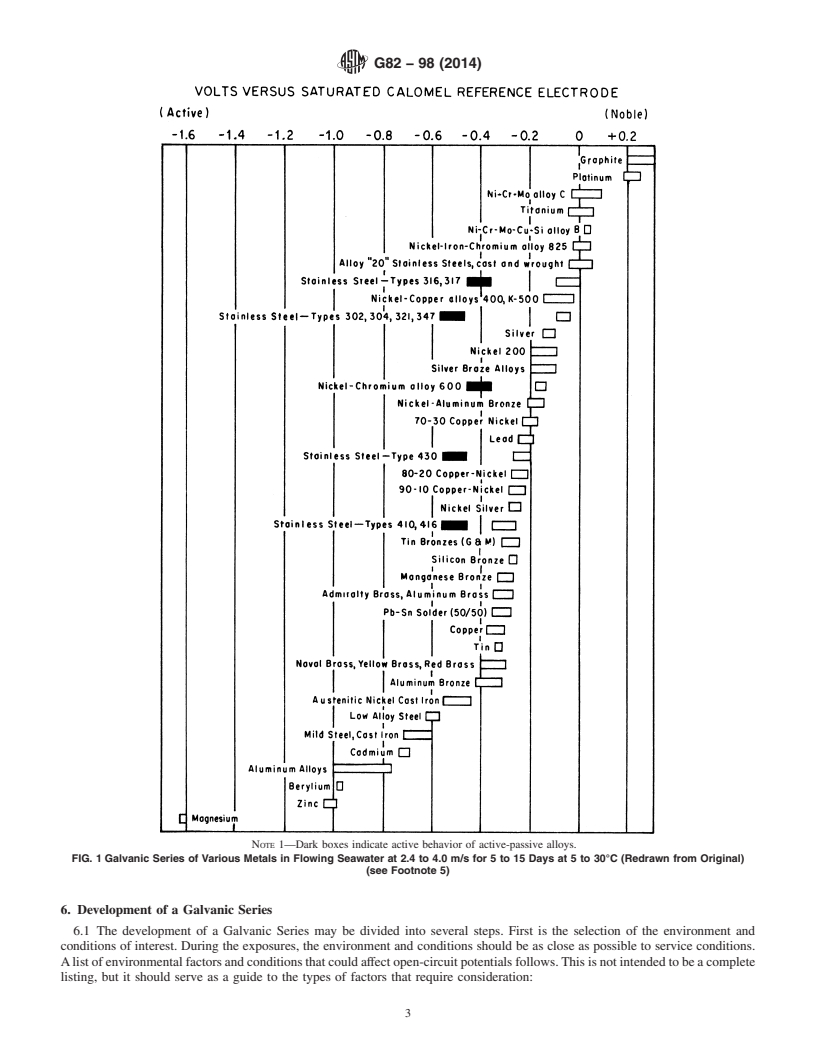
4.3.1 One type of Galvanic Series lists the metals of interest in order of their corrosion potentials, starting with the most active (electronegative) and proceeding in order to the most noble (electropositive). The potentials themselves (versus an appropriate reference half-cell) are listed so that the potential difference between metals in the series can be determined. This type of Galvanic Series has been put in graphical form as a series of bars displaying the range of potentials exhibited by the metal listed opposite each bar. Such a series is illustrated in Fig. 1.
4.3.2 The second type of galvanic series is similar to the first in that it lists the metals of interest in order of their corrosion potentials. The actual potentials themselves are not specified, however. Thus, only the relative position of materials in the series is known and not the magnitude of their potential difference. Such a series is shown in Fig. 2.
4.4 Use of a Galvanic Series:
4.4.1 Generally, upon coupling two metals in the G...
SCOPE
1.1 This guide covers the development of a galvanic series and its subsequent use as a method of predicting the effect that one metal can have upon another metal can when they are in electrical contact while immersed in an electrolyte. Suggestions for avoiding known pitfalls are included.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in Section 5.
General Information
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G82 − 98 (Reapproved 2014)
Standard Guide for
Development and Use of a Galvanic Series for Predicting
1
Galvanic Corrosion Performance
ThisstandardisissuedunderthefixeddesignationG82;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.2 active—the negative (decreasingly oxidizing) direction
of electrode potential.
1.1 This guide covers the development of a galvanic series
3.3 corrosionpotential—thepotentialofacorrodingsurface
and its subsequent use as a method of predicting the effect that
one metal can have upon another metal can when they are in in an electrolyte relative to a reference electrode measured
under open-circuit conditions.
electrical contact while immersed in an electrolyte. Sugges-
tions for avoiding known pitfalls are included.
3.4 galvanic corrosion—accelerated corrosion of a metal
because of an electrical contact with a more noble metal or
1.2 The values stated in SI units are to be regarded as
nonmetallic conductor in a corrosive electrolyte.
standard. No other units of measurement are included in this
standard.
3.5 galvanic series—a list of metals and alloys arranged
according to their relative corrosion potentials in a given
1.3 This standard does not purport to address all of the
environment.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
3.6 noble—thepositive(increasinglyoxidizing)directionof
priate safety and health practices and determine the applica-
electrode potential.
bility of regulatory limitations prior to use. Specific precau-
3.7 passive—the state of the metal surface characterized by
tionary statements are given in Section 5.
low corrosion rates in a potential region that is strongly
oxidizing for the metal.
2. Referenced Documents
3.8 polarization—the change from the open-circuit elec-
2
2.1 ASTM Standards:
trode potential as the result of the passage of current.
G3Practice for Conventions Applicable to Electrochemical
Measurements in Corrosion Testing
4. Significance and Use
G15TerminologyRelatingtoCorrosionandCorrosionTest-
3
4.1 When two dissimilar metals in electrical contact are
ing (Withdrawn 2010)
exposed to a common electrolyte, one of the metals can
G16Guide for Applying Statistics to Analysis of Corrosion
Data undergo increased corrosion while the other can show de-
creasedcorrosion.Thistypeofacceleratedcorrosionisreferred
G71Guide for Conducting and Evaluating Galvanic Corro-
sion Tests in Electrolytes toasgalvaniccorrosion.Becausegalvaniccorrosioncanoccur
at a high rate, it is important that a means be available to alert
3. Terminology
the user of products or equipment that involve the use of
dissimilar metal combinations in an electrolyte of the possible
3.1 Definitions of terms used in this guide are from Termi-
effects of galvanic corrosion.
nology G15.
4.2 Onemethodthatisusedtopredicttheeffectsofgalvanic
corrosion is to develop a galvanic series by arranging a list of
1
This guide is under the jurisdiction ofASTM Committee G01 on Corrosion of
the materials of interest in order of observed corrosion poten-
Metalsand is the direct responsibility of Subcommittee G01.11 on Electrochemical
tials in the environment and conditions of interest. The metal
Measurements in Corrosion Testing.
that will suffer increased corrosion in a galvanic couple in that
Current edition approved May 1, 2014. Published May 2014. Originally
approved in 1983. Last previous edition approved in 2009 as G82–98(2009). DOI:
environmentcanthenbepredictedfromtherelativepositionof
10.1520/G0082-98R14.
the two metals in the series.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
4.3 Types of Galvanic Series:
Standards volume information, refer to the standard’s Document Summary page on
4.3.1 OnetypeofGalvanicSeriesliststhemetalsofinterest
the ASTM website.
3
in order of their corrosion potentials, starting with the most
The last approved version of this historical standard is referenced on
www.astm.org. active (electronegative) and proceeding in order to the most
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
G82 − 98 (2014)
noble (electropositive). The potentials themselves (versus an series of bars displaying the range of potentials exhibited by
appropriate reference half-cell) are listed so that the potential themetallistedoppositeeachbar.Suchaseriesisillustratedin
differencebetweenmetalsinthese
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: G82 − 98 (Reapproved 2009) G82 − 98 (Reapproved 2014)
Standard Guide for
Development and Use of a Galvanic Series for Predicting
1
Galvanic Corrosion Performance
This standard is issued under the fixed designation G82; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This guide covers the development of a galvanic series and its subsequent use as a method of predicting the effect that one
metal can have upon another metal can when they are in electrical contact while immersed in an electrolyte. Suggestions for
avoiding known pitfalls are included.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use. Specific precautionary statements are given in Section 5.
2. Referenced Documents
2
2.1 ASTM Standards:
G3 Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing
3
G15 Terminology Relating to Corrosion and Corrosion Testing (Withdrawn 2010)
G16 Guide for Applying Statistics to Analysis of Corrosion Data
G71 Guide for Conducting and Evaluating Galvanic Corrosion Tests in Electrolytes
3. Terminology
3.1 Definitions of terms used in this guide are from Terminology G15.
3.2 active—the negative (decreasingly oxidizing) direction of electrode potential.
3.3 corrosion potential—the potential of a corroding surface in an electrolyte relative to a reference electrode measured under
open-circuit conditions.
3.4 galvanic corrosion—accelerated corrosion of a metal because of an electrical contact with a more noble metal or nonmetallic
conductor in a corrosive electrolyte.
3.5 galvanic series—a list of metals and alloys arranged according to their relative corrosion potentials in a given environment.
3.6 noble—the positive (increasingly oxidizing) direction of electrode potential.
3.7 passive—the state of the metal surface characterized by low corrosion rates in a potential region that is strongly oxidizing
for the metal.
3.8 polarization—the change from the open-circuit electrode potential as the result of the passage of current.
4. Significance and Use
4.1 When two dissimilar metals in electrical contact are exposed to a common electrolyte, one of the metals can undergo
increased corrosion while the other can show decreased corrosion. This type of accelerated corrosion is referred to as galvanic
corrosion. Because galvanic corrosion can occur at a high rate, it is important that a means be available to alert the user of products
or equipment that involve the use of dissimilar metal combinations in an electrolyte of the possible effects of galvanic corrosion.
1
This guide is under the jurisdiction of ASTM Committee G01 on Corrosion of Metalsand is the direct responsibility of Subcommittee G01.11 on Electrochemical
Measurements in Corrosion Testing.
Current edition approved May 1, 2009May 1, 2014. Published May 2009May 2014. Originally approved in 1983. Last previous edition approved in 20032009 as
G82–98(2003).G82–98(2009). DOI: 10.1520/G0082-98R09.10.1520/G0082-98R14.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
The last approved version of this historical standard is referenced on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
G82 − 98 (2014)
4.2 One method that is used to predict the effects of galvanic corrosion is to develop a galvanic series by arranging a list of the
materials of interest in order of observed corrosion potentials in the environment and conditions of interest. The metal that will
suffer increased corrosion in a galvanic couple in that environment can then be predicted from the relative position of the two
metals in the series.
4.3 Types of Galvanic Series:
4.3.1 One type
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.