ASTM F748-06(2010)
(Practice)Standard Practice for Selecting Generic Biological Test Methods for Materials and Devices
Standard Practice for Selecting Generic Biological Test Methods for Materials and Devices
SIGNIFICANCE AND USE
The objective of this practice is to recommend sufficient biological testing to establish a reasonable level of confidence concerning the biological response to a material or device, while at the same time avoiding unnecessary testing.
This practice is intended to provide guidance to the materials investigator in selecting the proper procedures to be carried out for the screening of new or modified materials. Because each material and each implant situation involves its own unique circumstances, these recommendations should be modified as necessary and do not constitute the only testing that will be required for a material nor should these guidelines be interpreted as minimum requirements for any particular situation. While an attempt has been made to provide recommendation for different implant circumstances, some of the recommended testing may not be necessary or reasonable for a specific material or application.
SCOPE
1.1 This practice recommends generic biological test methods for materials and devices according to end-use applications. While chemical testing for extractable additives and residual monomers or residues from processing aids is necessary for most implant materials, such testing is not included as part of this practice. The reader is cautioned that the area of materials biocompatibility testing is a rapidly evolving field, and improved methods are evolving rapidly, so this practice is by necessity only a guideline. A thorough knowledge of current techniques and research is critical to a complete evaluation of new materials.
1.2 These test protocols are intended to apply to materials and medical devices for human application. Biological evaluation of materials and devices, and related subjects such as pyrogen testing, batch testing of production lots, and so on, are also discussed. Tests include those performed on materials, end products, and extracts. Rationale and comments on current state of the art are included for all test procedures described.
1.3 The biocompatibility of materials used in single or multicomponent medical devices for human use depends to a large degree on the particular nature of the end-use application. Biological reactions that are detrimental to the success of a material in one device application may have little or no bearing on the successful use of the material for a different application. It is, therefore, not possible to specify a set of biocompatibility test methods which will be necessary and sufficient to establish biocompatibility for all materials and applications.
1.4 The evaluation of tissue engineered medical products (TEMPs) may, in some cases, involve different or additional testing beyond those suggested for non-tissue-based materials and devices. Where appropriate, these differences are discussed in this practice and additional tests described.
1.5 The ethical use of research animals places the obligation on the individual investigator to determine the most efficient methods for performing the necessary testing without undue use of animals. Where adequate prior data exists to substantiate certain types of safety information, these guidelines should not be interpreted to mean that testing should be unnecessarily repeated.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F748 − 06(Reapproved 2010)
Standard Practice for
Selecting Generic Biological Test Methods for Materials and
Devices
ThisstandardisissuedunderthefixeddesignationF748;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope methods for performing the necessary testing without undue
useofanimals.Whereadequatepriordataexiststosubstantiate
1.1 This practice recommends generic biological test meth-
certain types of safety information, these guidelines should not
ods for materials and devices according to end-use applica-
be interpreted to mean that testing should be unnecessarily
tions. While chemical testing for extractable additives and
repeated.
residual monomers or residues from processing aids is neces-
1.6 This standard does not purport to address all of the
sary for most implant materials, such testing is not included as
safety concerns, if any, associated with its use. It is the
part of this practice. The reader is cautioned that the area of
responsibility of the user of this standard to establish appro-
materials biocompatibility testing is a rapidly evolving field,
priate safety and health practices and determine the applica-
and improved methods are evolving rapidly, so this practice is
bility of regulatory limitations prior to use.
bynecessityonlyaguideline.Athoroughknowledgeofcurrent
techniques and research is critical to a complete evaluation of
2. Referenced Documents
new materials.
2.1 ASTM Standards:
1.2 These test protocols are intended to apply to materials
E1202 Guide for Development of MicronucleusAssay Stan-
and medical devices for human application. Biological evalu-
dards
ation of materials and devices, and related subjects such as
E1262 Guide for Performance of Chinese Hamster Ovary
pyrogen testing, batch testing of production lots, and so on, are
Cell/Hypoxanthine Guanine Phosphoribosyl Transferase
also discussed.Tests include those performed on materials, end
Gene Mutation Assay
products, and extracts. Rationale and comments on current
E1263 Guide for Conduct of Micronucleus Assays in Mam-
state of the art are included for all test procedures described.
malian Bone Marrow Erythrocytes
1.3 The biocompatibility of materials used in single or
E1280 Guide for Performing the Mouse Lymphoma Assay
multicomponent medical devices for human use depends to a
for Mammalian Cell Mutagenicity
large degree on the particular nature of the end-use application.
E1397 Practice forIn Vitro Rat Hepatocyte DNA Repair
Biological reactions that are detrimental to the success of a
Assay
material in one device application may have little or no bearing
E1398 Practice forIn Vivo Rat Hepatocyte DNA Repair
on the successful use of the material for a different application.
Assay
It is, therefore, not possible to specify a set of biocompatibility
F619 Practice for Extraction of Medical Plastics
test methods which will be necessary and sufficient to establish
F719 Practice for Testing Biomaterials in Rabbits for Pri-
biocompatibility for all materials and applications.
mary Skin Irritation
1.4 The evaluation of tissue engineered medical products
F720 Practice forTesting Guinea Pigs for ContactAllergens:
(TEMPs) may, in some cases, involve different or additional
Guinea Pig Maximization Test
testing beyond those suggested for non-tissue-based materials
F749 Practice for Evaluating Material Extracts by Intracuta-
anddevices.Whereappropriate,thesedifferencesarediscussed
neous Injection in the Rabbit
in this practice and additional tests described.
F750 Practice for Evaluating Material Extracts by Systemic
Injection in the Mouse
1.5 The ethical use of research animals places the obligation
F756 Practice for Assessment of Hemolytic Properties of
on the individual investigator to determine the most efficient
Materials
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
Surgical Materials and Devicesand is direct responsibility of Subcommittee F04.16
on Biocompatibility Test Methods. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved June 1, 2010. Published September 2010. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1982. Last previous edition approved in 2006 as F748 – 06. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/F0748-06R10. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F748 − 06 (2010)
F763 Practice for Short-Term Screening of Implant Materi- 3. Summary of Practice
als
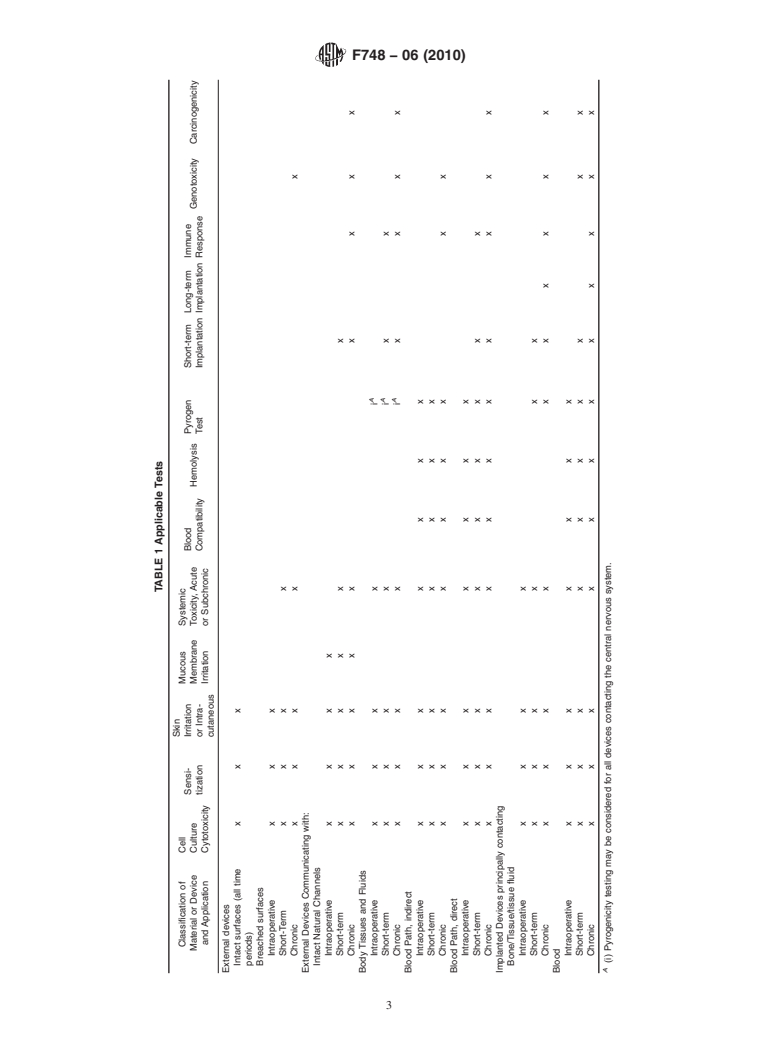
3.1 Amatrix listing biological test methods versus materials
F813 Practice for Direct Contact Cell Culture Evaluation of
(devices) and their applications is included in Table 1. The
Materials for Medical Devices
expected duration of use of the device is also considered.
F895 TestMethodforAgarDiffusionCellCultureScreening
Intraoperative is less than 24 h, short-term is up to and
for Cytotoxicity
including30days,chronicisgreaterthan30days.Theposition
F981 Practice for Assessment of Compatibility of Biomate-
of row and column intersection is marked to indicate whether
rials for Surgical Implants with Respect to Effect of
thetestisrecommendedforamaterialordeviceforthespecific
Materials on Muscle and Bone
application indicated. The terms relating to device or material
F1027 Practice for Assessment of Tissue and Cell Compat-
type and application are addressed in Section 5. Discussion of
ibility of Orofacial Prosthetic Materials and Devices
applicability, current state of the art, and rationale for indi-
F1408 Practice for Subcutaneous Screening Test for Implant
vidual test methods also appears in that section.
Materials
4. Significance and Use
F1439 Guide for Performance of Lifetime Bioassay for the
Tumorigenic Potential of Implant Materials
4.1 The objective of this practice is to recommend sufficient
F1877 Practice for Characterization of Particles biological testing to establish a reasonable level of confidence
F1903 Practice for Testing For Biological Responses to
concerning the biological response to a material or device,
Particles In Vitro while at the same time avoiding unnecessary testing.
F1904 Practice for Testing the Biological Responses to
4.2 This practice is intended to provide guidance to the
Particles in vivo
materials investigator in selecting the proper procedures to be
F1905 Practice For Selecting Tests for Determining the
carried out for the screening of new or modified materials.
Propensity of Materials to Cause Immunotoxicity (With-
Because each material and each implant situation involves its
drawn 2011)
own unique circumstances, these recommendations should be
F1906 Practice for Evaluation of Immune Responses In
modified as necessary and do not constitute the only testing
BiocompatibilityTestingUsingELISATests,Lymphocyte
that will be required for a material nor should these guidelines
Proliferation, and Cell Migration (Withdrawn 2011)
be interpreted as minimum requirements for any particular
F1983 Practice for Assessment of Compatibility of
situation. While an attempt has been made to provide recom-
Absorbable/Resorbable Biomaterials for ImplantApplica-
mendation for different implant circumstances, some of the
tions
recommended testing may not be necessary or reasonable for a
F1984 Practice for Testing for Whole Complement Activa-
specific material or application.
tion in Serum by Solid Materials
5. Classification of Materials and Devices by End-Use
F2065 Practice for Testing forAlternative Pathway Comple-
Applications
ment Activation in Serum by Solid Materials
F2147 Practice for Guinea Pig: Split Adjuvant and Closed
5.1 General:
Patch Testing for Contact Allergens
5.1.1 When new materials are sought for a medical appli-
F2148 Practice for Evaluation of Delayed Contact Hyper-
cation for use on humans, the material(s) may comprise the
sensitivity Using the Murine Local Lymph Node Assay
whole final device product, or may be one of many component
(LLNA)
materials in the device. The first step is a thorough literature
F2151 Practice for Assessment of White Blood Cell Mor-
search for previous use of the material or biocompatibility
phology After Contact with Materials (Withdrawn 2007)
testing studies to ensure that it has not been known to produce
F2382 Test Method forAssessment of Intravascular Medical an adverse biological response that exceeds the expected
Device Materials on Partial Thromboplastin Time (PTT) benefit in the use of the device. Note that the final fabricated
product may differ chemically, physically, or biologically from
2.2 Other Referenced Documents:
the raw materials used to fabricate the product due to process-
ISO/AAMI/ANSI 10993-1 Biological Testing of Medical
ingandthishastobeconsideredwhendesigningtestprotocols.
and Dental Materials and Devices - Part 1: Guidance on
For some devices, it may be necessary or desirable to take
Selection of Tests
material test samples directly from the final device product.
EN 30993–1 Biological Testing of Medical and Dental
Samples should be fully representative of the finished product
Materials and Devices - Part 1: Guidance on Selection of
in terms of processing, cleaning, packaging, sterilization, and
Tests
anyotherproceduresthatareperformedonthematerialsbefore
General Program Memorandum #G95-1 FDA
the device is used.
Immunotoxicity Testing Guidance-FDA
5.1.2 At this point, preliminary material screening may be
employed, depending on the expertise of the organizations
evaluating the materials. Since preliminary screening is nor-
The last approved version of this historical standard is referenced on
mally an option to minimize the economic impact of a
www.astm.org.
candidate material failing final biological tests after extensive
Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
time and effort, it is not a required procedure. The investigator
4th Floor, New York, NY 10036, http://www.ansi.org.
Available from CDRH, 5600 Fishers Ln., Rockville, MD 20857. should be aware that, should an adverse tissue response be
F748 − 06 (2010)
TABLE 1 Applicable Tests
Skin
Classification of Cell Mucous Systemic
Sensi- Irritation Blood Pyrogen Short-term Long-term Immune
Material or Device Culture Membrane Toxicity, Acute Hemolysis Genotoxicity Carcinogenicity
tization or Intra- Compatibility Test Implantation Implantation Response
and Application Cytotoxicity Irritation or Subchronic
cutaneous
External devices
Intact surfaces (all time xx x
periods)
Breached surfaces
Intraoperative x x x
Short-Term x x x x
Chronic x x x x x
External Devices Communicating with:
Intact Natural Channels
Intraoperative x x x x
Short-term x x x x x x
Chronic x x x x x x x x x
Body Tissues and Fluids
A
Intraoperative x x x x i
A
Short-term x x x x i xx
A
Chronic x x x x i xxx x
Blood Path, indirect
Intraoperative x x x x x x x
Short-term x x x x x x x
Chronic x x x x x x x x x
Blood Path, direct
Intraoperative x x x x x x x
Short-term x x x x x x x x x
Chronic x x x x x x x x x x x
Implanted Devices principally contacting
Bone/Tissue/tissue fluid
Intraoperative x x x x
Short-term x x x x x x
Chronic x x x x x x x x x x
Blood
Intraoperative x x x x x x x
Short-term x x x x x x x x x x
Chronic x x x x x x x x x x x x
A
(i) Pyrogenicity testing may be considered for all devices contacting the central nervous system.
F748 − 06 (2010)
observed with a final product, it may be impossible to 5.3.2.2 Short-term(uptoandincluding30days)—examples
determine which component or process is responsible without include cranial calipers, perfusion apparatus, drainage
these initial screening tests. apparatus, stabilizing orthopedic devices, and any parts of
5.1.3 This practice addresses two dimensions of tissue- ancillary equipment that are in contact with material entering
material interactions: duration and tissue type. A third the body.
dimension, which should be considered, is the relative size 5.3.2.3 Chronic (>30 days)—examples include percutane-
difference between the host and the material, that is, to how ous electrodes, active penetrating electrodes, stapedectomy
much material surface area is the host exposed. The material prostheses, partial and total ossicular replacement prostheses,
surface area to body weight ratio may become a significant or tympanoplasty ventilation tubes.
factor for porous materials, and devices of repeated short-term 5.3.3 Blood Path, Indirect—Products contacting blood path
applications (for example, dialysis products). While this prac- at one point for usually less than 24 hours, and serves as a
ticedoesnotaddresstheissueof“intensityfactor”ofincreased conduit for fluid entry into the vascular system. Examples
surface area, the biocompatibility testing facility personnel include solution administration sets, extension sets, transfer
should consider it in their material screening and testing sets, or blood administration sets.
protocol design. 5.3.3.1 Products that are used for >24 hours or that are used
5.1.4 For the purposes of this practice, devices and the repeatedly in the same patient will be considered as chronic
materialsthatcomprisethemareclassifiedastoend-usehuman usage and should undergo extended testing.
application as outlined in 5.2-5.4. 5.3.4 Blood, Path, Direct—Single recirculating blood expo-
5.1.5 In general, the testing for tissue engineered medical sure or product is in blood path generally less than 24 hours.
products (TEMPs) should address the same issues specific to Examplesincludeintravenouscatheters,oxygenators,extracor-
the type, location, and duration of use as other medical devices poreal oxygenator tubing and accessories.
and products. The selection of additional testing for compat- 5.3.5 Blood Path, Direct, Short Term, or Chronic, or re-
ibility criteria unique to these type of products should be peated exposure—Examples include dialyzers or dialysis tub-
conducted with these recommendations in mind. ing and accessories, shunts.
5.1.6 When testing materials that are intended to degrade
5.4 Implanted Long-Term Devices:
and/or be metabolized while implanted in the body (both
5.4.1 Devices Principally Contacting Bones—examples in-
synthetic and TEMPs), consideration should be given to the
cludd orthopedic pins, screws, replacement joints, bone
degradation or metabolic products and appropriate modifica-
prostheses, cements, o
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.