ASTM F748-16
(Practice)Standard Practice for Selecting Generic Biological Test Methods for Materials and Devices
Standard Practice for Selecting Generic Biological Test Methods for Materials and Devices
SIGNIFICANCE AND USE
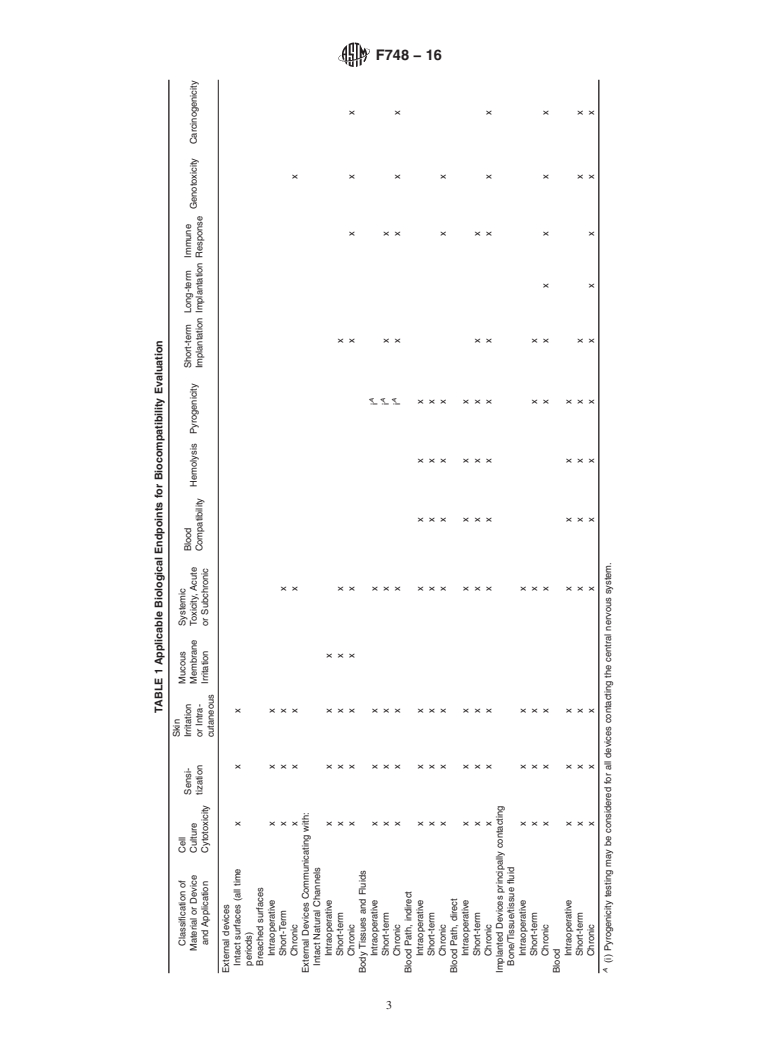
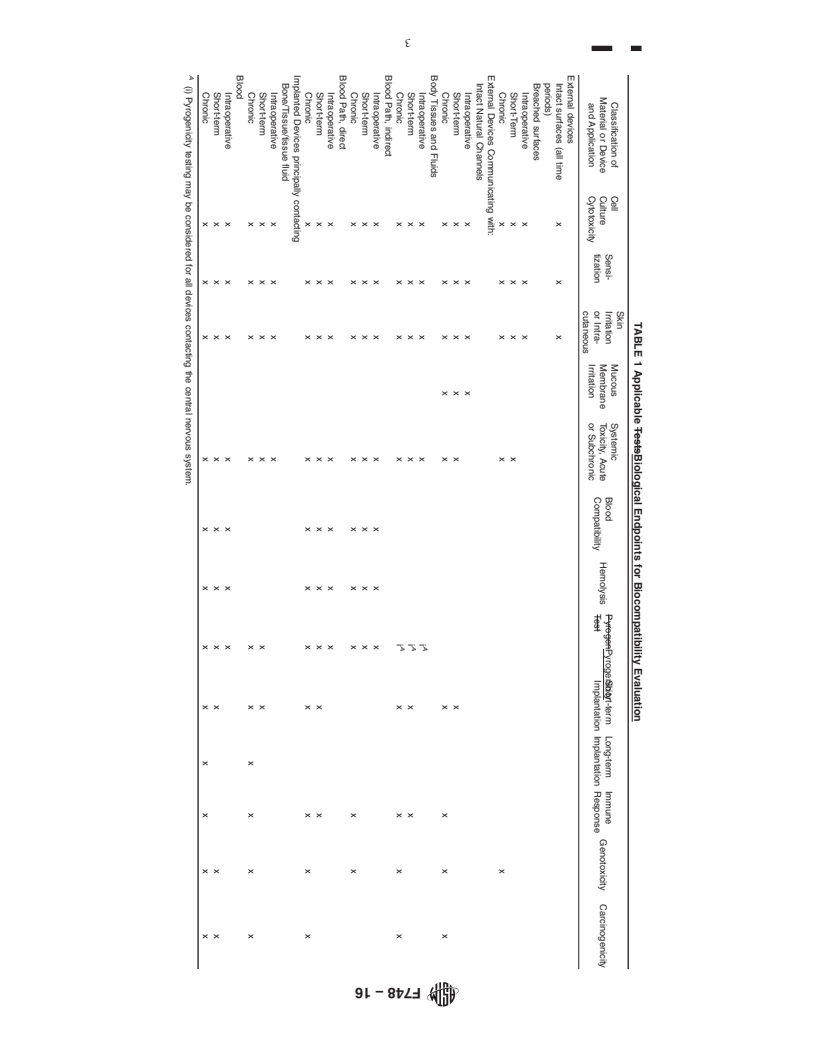
4.1 The objective of this practice is to recommend appropriate biological endpoint assessments (which may or may not require testing) to establish a reasonable level of confidence concerning the biological response to a material or device, while at the same time avoiding unnecessary testing.
4.2 This practice is intended to provide guidance to the materials investigator in selecting the proper procedures to be carried out for the screening of new or modified materials. Because each material and each implant situation involves its own unique circumstances, these recommendations should be modified as necessary and do not constitute the only assessment that will be required for a material. Nor should these guidelines be interpreted as minimum requirements for any particular situation. While an attempt has been made to provide recommendation for different implant circumstances, some of the recommended assessment may not be necessary or reasonable for a specific material or application.
SCOPE
1.1 This practice recommends generic biological test methods for materials and devices according to end-use applications. While chemical testing for extractable additives and residual monomers or residues from processing aids is necessary for most implant materials, such testing is not included as part of this practice. The reader is cautioned that the area of materials biocompatibility testing is a rapidly evolving field, and improved methods are evolving rapidly, so this practice is by necessity only a guideline. A thorough knowledge of current techniques and research is critical to a complete evaluation of new materials.
1.2 These test protocols are intended to apply to materials and medical devices for human application. Biological evaluation of materials and devices, and related subjects such as pyrogen testing, batch testing of production lots, and so on, are also discussed. Tests include those performed on materials, end products, and extracts. Rationale and comments on current state of the art are included for all test procedures described.
1.3 The biocompatibility of materials used in single or multicomponent medical devices for human use depends to a large degree on the particular nature of the end-use application. Biological reactions that are detrimental to the success of a material in one device application may have little or no bearing on the successful use of the material for a different application. It is, therefore, not possible to specify a set of biocompatibility test methods which will be necessary and sufficient to establish biocompatibility for all materials and applications.
1.4 The evaluation of tissue engineered medical products (TEMPs) may, in some cases, involve different or additional testing beyond those suggested for non-tissue-based materials and devices. Where appropriate, these differences are discussed in this practice and additional tests described.
1.5 The ethical use of research animals places the obligation on the individual investigator to determine the most efficient methods for performing the necessary testing without undue use of animals. Where adequate prior data exists to substantiate certain types of safety information, these guidelines should not be interpreted to mean that testing should be unnecessarily repeated.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F748 − 16
Standard Practice for
Selecting Generic Biological Test Methods for Materials and
1
Devices
ThisstandardisissuedunderthefixeddesignationF748;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginal
adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.Asuperscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope methods for performing the necessary testing without undue
useofanimals.Whereadequatepriordataexiststosubstantiate
1.1 This practice recommends generic biological test meth-
certain types of safety information, these guidelines should not
ods for materials and devices according to end-use applica-
be interpreted to mean that testing should be unnecessarily
tions. While chemical testing for extractable additives and
repeated.
residual monomers or residues from processing aids is neces-
1.6 This standard does not purport to address all of the
sary for most implant materials, such testing is not included as
safety concerns, if any, associated with its use. It is the
part of this practice. The reader is cautioned that the area of
responsibility of the user of this standard to establish appro-
materials biocompatibility testing is a rapidly evolving field,
priate safety and health practices and determine the applica-
and improved methods are evolving rapidly, so this practice is
bility of regulatory limitations prior to use.
bynecessityonlyaguideline.Athoroughknowledgeofcurrent
techniques and research is critical to a complete evaluation of
2. Referenced Documents
new materials.
2
2.1 ASTM Standards:
1.2 These test protocols are intended to apply to materials
E1262 Guide for Performance of Chinese Hamster Ovary
and medical devices for human application. Biological evalu-
Cell/Hypoxanthine Guanine Phosphoribosyl Transferase
ation of materials and devices, and related subjects such as
Gene Mutation Assay
pyrogen testing, batch testing of production lots, and so on, are
F619 Practice for Extraction of Medical Plastics
alsodiscussed.Testsincludethoseperformedonmaterials,end
F719 Practice for Testing Biomaterials in Rabbits for Pri-
products, and extracts. Rationale and comments on current
mary Skin Irritation
state of the art are included for all test procedures described.
F720 PracticeforTestingGuineaPigsforContactAllergens:
1.3 The biocompatibility of materials used in single or
Guinea Pig Maximization Test
multicomponent medical devices for human use depends to a
F749 Practice for Evaluating Material Extracts by Intracuta-
largedegreeontheparticularnatureoftheend-useapplication.
neous Injection in the Rabbit
Biological reactions that are detrimental to the success of a
F750 Practice for Evaluating Material Extracts by Systemic
materialinonedeviceapplicationmayhavelittleornobearing
Injection in the Mouse
on the successful use of the material for a different application.
F756 Practice for Assessment of Hemolytic Properties of
It is, therefore, not possible to specify a set of biocompatibility
Materials
test methods which will be necessary and sufficient to establish
F763 Practice for Short-Term Screening of Implant Materi-
biocompatibility for all materials and applications.
als
F813 Practice for Direct Contact Cell Culture Evaluation of
1.4 The evaluation of tissue engineered medical products
Materials for Medical Devices
(TEMPs) may, in some cases, involve different or additional
F895 TestMethodforAgarDiffusionCellCultureScreening
testing beyond those suggested for non-tissue-based materials
for Cytotoxicity
anddevices.Whereappropriate,thesedifferencesarediscussed
F981 Practice for Assessment of Compatibility of Biomate-
in this practice and additional tests described.
rials for Surgical Implants with Respect to Effect of
1.5 Theethicaluseofresearchanimalsplacestheobligation
Materials on Muscle and Bone
on the individual investigator to determine the most efficient
F1027 Practice for Assessment of Tissue and Cell Compat-
ibility of Orofacial Prosthetic Materials and Devices
1
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
Surgical Materials and Devicesand is direct responsibility of Subcommittee F04.16
2
on Biocompatibility Test Methods. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved April 1, 2016. Published May 2016. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1982. Last previous edition approved in 2010 as F748 – 06 (2010). Standards volume information, refer to the standard’s Document Summary page on
DOI: 10.1520/F0748-16. th
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F748 − 06 (Reapproved 2010) F748 − 16
Standard Practice for
Selecting Generic Biological Test Methods for Materials and
1
Devices
This standard is issued under the fixed designation F748; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This practice recommends generic biological test methods for materials and devices according to end-use applications.
While chemical testing for extractable additives and residual monomers or residues from processing aids is necessary for most
implant materials, such testing is not included as part of this practice. The reader is cautioned that the area of materials
biocompatibility testing is a rapidly evolving field, and improved methods are evolving rapidly, so this practice is by necessity only
a guideline. A thorough knowledge of current techniques and research is critical to a complete evaluation of new materials.
1.2 These test protocols are intended to apply to materials and medical devices for human application. Biological evaluation of
materials and devices, and related subjects such as pyrogen testing, batch testing of production lots, and so on, are also discussed.
Tests include those performed on materials, end products, and extracts. Rationale and comments on current state of the art are
included for all test procedures described.
1.3 The biocompatibility of materials used in single or multicomponent medical devices for human use depends to a large
degree on the particular nature of the end-use application. Biological reactions that are detrimental to the success of a material in
one device application may have little or no bearing on the successful use of the material for a different application. It is, therefore,
not possible to specify a set of biocompatibility test methods which will be necessary and sufficient to establish biocompatibility
for all materials and applications.
1.4 The evaluation of tissue engineered medical products (TEMPs) may, in some cases, involve different or additional testing
beyond those suggested for non-tissue-based materials and devices. Where appropriate, these differences are discussed in this
practice and additional tests described.
1.5 The ethical use of research animals places the obligation on the individual investigator to determine the most efficient
methods for performing the necessary testing without undue use of animals. Where adequate prior data exists to substantiate certain
types of safety information, these guidelines should not be interpreted to mean that testing should be unnecessarily repeated.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2
2.1 ASTM Standards:
3
E1202 Guide for Development of Micronucleus Assay Standards (Withdrawn 2013)
E1262 Guide for Performance of Chinese Hamster Ovary Cell/Hypoxanthine Guanine Phosphoribosyl Transferase Gene
Mutation Assay
3
E1263 Guide for Conduct of Micronucleus Assays in Mammalian Bone Marrow Erythrocytes (Withdrawn 2014)
3
E1280 Guide for Performing the Mouse Lymphoma Assay for Mammalian Cell Mutagenicity (Withdrawn 2014)
3
E1397 Practice for In Vitro Rat Hepatocyte DNA Repair Assay (Withdrawn 2013)
3
E1398 Practice for In Vivo Rat Hepatocyte DNA Repair Assay (Withdrawn 2013)
1
This practice is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devicesand is direct responsibility of Subcommittee F04.16 on
Biocompatibility Test Methods.
Current edition approved June 1, 2010April 1, 2016. Published September 2010May 2016. Originally approved in 1982. Last previous edition approved in 20062010 as
F748 – 06.F748 – 06 (2010). DOI: 10.1520/F0748-06R10.10.1520/F0748-16.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
3
The last approved version of this historical standard is referenced on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.