ASTM G1-90(1999)e1
(Practice)Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens
Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens
SCOPE
1.1 This practice covers suggested procedures for preparing bare, solid metal specimens for tests, for removing corrosion products after the test has been completed, and for evaluating the corrosion damage that has occurred. Emphasis is placed on procedures related to the evaluation of corrosion by mass loss and pitting measurements. Note 1-In many cases the corrosion product on the reactive metals titanium and zirconium is a hard and tightly bonded oxide that defies removal by chemical or ordinary mechanical means. In many such cases, corrosion rates are established by mass gain rather than mass loss.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific precautionary statements, see Notes 1 and 6.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
e1
Designation:G1–90 (Reapproved 1999)
Standard Practice for
Preparing, Cleaning, and Evaluating Corrosion Test
Specimens
This standard is issued under the fixed designation G 1; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—Editorial corrections were made throughout in January 1999.
1. Scope G 31 Practice for Laboratory Immersion Corrosion Testing
of Metals
1.1 This practice covers suggested procedures for preparing
G 33 Practice for Recording Data from Atmospheric Cor-
bare, solid metal specimens for tests, for removing corrosion
rosion Tests of Metallic-Coated Steel Specimens
products after the test has been completed, and for evaluating
G 46 Guide for Examination and Evaluation of Pitting
the corrosion damage that has occurred. Emphasis is placed on
Corrosion
procedures related to the evaluation of corrosion by mass loss
G 50 Practice for Conducting Atmospheric Corrosion Tests
and pitting measurements.
on Metals
NOTE 1—Caution: In many cases the corrosion product on the reactive
G 78 Guide for Crevice Corrosion Testing of Iron Base and
metals titanium and zirconium is a hard and tightly bonded oxide that
Nickel Base Stainless Alloys in Seawater and Other
defies removal by chemical or ordinary mechanical means. In many such
Chloride-Containing Aqueous Environments
cases, corrosion rates are established by mass gain rather than mass loss.
1.2 This standard does not purport to address all of the
3. Terminology
safety concerns, if any, associated with its use. It is the
3.1 See Terminology G 15 for terms used in this practice.
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
4. Significance and Use
bility of regulatory limitations prior to use. For specific
4.1 The procedures given are designed to remove corrosion
precautionary statements, see Note 1 and Note 6.
products without significant removal of base metal. This allows
an accurate determination of the mass loss of the metal or alloy
2. Referenced Documents
that occurred during exposure to the corrosive environment.
2.1 ASTM Standards:
4.2 These procedures, in some cases, may apply to metal
A 262 Practices for Detecting Susceptibility to Intergranu-
coatings. However, possible effects from the substrate must be
lar Attack in Austenitic Stainless Steels
considered.
D 1193 Specification for Reagent Water
D 1384 Test Method for Corrosion Test for Engine Coolants
5. Reagents and Materials
in Glassware
5.1 Purity of Reagents—Reagent grade chemicals shall be
D 2776 Test Methods for Corrosivity of Water in the Ab-
used in all tests. Unless otherwise indicated, it is intended that
sence of Heat Transfer (Electrical Methods)
all reagents conform to the specifications of the Committee on
G 15 Terminology Relating to Corrosion and Corrosion
Analytical Reagents of the American Chemical Society where
Testing 7
such specifications are available. Other grades may be used,
G 16 Guide for Applying Statistics to Analysis of Corrosion
provided it is first ascertained that the reagent is of sufficiently
Data
high purity to permit its use without lessening the accuracy of
the determination.
5.2 Purity of Water—Unless otherwise indicated, references
to water shall be understood to mean reagent water as defined
This practice is under the jurisdiction of ASTM Committee G-1 on Corrosion
of Metals and is the direct responsibility of Subcommittee G01.05 on Laboratory
by Type IV of Specification D 1193.
Corrosion Tests.
Current edition approved March 30, 1990. Published May 1990. Originally
published as G 1 – 67. Last previous edition G1–88.
2 7
Annual Book of ASTM Standards, Vol 01.03. Reagent Chemicals, American Chemical Society Specifications, American
Annual Book of ASTM Standards, Vol 11.01. Chemical Society, Washington, DC. For suggestions on the testing of reagents not
Annual Book of ASTM Standards, Vol 15.05. listed by the American Chemical Society, see Analar Standards for Laboratory
Discontinued—Replaced by Guide G 96. See 1990 Annual Book of ASTM Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
Standards, Vol 03.02. and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
Annual Book of ASTM Standards, Vol 03.02. MD.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
G1
6. Methods for Preparing Specimens for Test should be abraded to remove burrs.
6.3.4 Rinse thoroughly, hot air dry, and store in desiccator.
6.1 For laboratory corrosion tests that simulate exposure to
6.4 When specimen preparation changes the metallurgical
service environments, a commercial surface, closely resem-
condition of the metal, other methods should be chosen or the
bling the one that would be used in service, will yield the most
metallurgical condition must be corrected by subsequent treat-
meaningful results.
ment. For example, shearing a specimen to size will cold work
6.2 It is desirable to mark specimens used in corrosion tests
and may possibly fracture the edges. Edges should be ma-
with a unique designation during preparation. Several tech-
chined.
niques may be used depending on the type of specimen and
6.5 The clean, dry specimens should be measured and
test.
weighed. Dimensions determined to the third significant figure
6.2.1 Stencil or Stamp—Most metallic specimens may be
and mass determined to the fifth significant figure are sug-
marked by stenciling, that is, imprinting the designation code
gested. When more significant figures are available on the
into the metal surface using hardened steel stencil stamps hit
measuring instruments, they should be recorded.
with a hammer. The resulting imprint will be visible even after
substantial corrosion has occurred. However, this procedure
7. Methods for Cleaning After Testing
introduces localized strained regions and the possibility of
7.1 Corrosion product removal procedures can be divided
superficial iron contamination in the marked area.
into three general categories: mechanical, chemical, and elec-
6.2.2 Electric engraving by means of a vibratory marking
trolytic.
tool may be used when the extent of corrosion damage is
7.1.1 An ideal procedure should remove only corrosion
known to be small. However, this approach to marking is much
products and not result in removal of any base metal. To
more susceptible to having the marks lost as a result of
determine the mass loss of the base metal when removing
corrosion damage during testing.
corrosion products, replicate uncorroded control specimens
6.2.3 Edge notching is especially applicable when extensive
should be cleaned by the same procedure being used on the test
corrosion and accumulation of corrosion products is antici-
specimen. By weighing the control specimen before and after
pated. Long term atmospheric tests and sea water immersion
cleaning, the extent of metal loss resulting from cleaning can
tests on steel alloys are examples where this approach is
be utilized to correct the corrosion mass loss.
applicable. It is necessary to develop a code system when using
edge notches.
NOTE 5—It is desirable to scrape samples of corrosion products before
6.2.4 Drilled holes may also be used to identify specimens
using any chemical techniques to remove them. These scrapings can then
when extensive metal loss, accumulation of corrosion products,
be subjected to various forms of analyses, including perhaps X-ray
diffraction to determine crystal forms as well as chemical analyses to look
or heavy scaling is anticipated. Drilled holes may be simpler
for specific corrodants, such as chlorides. All of the chemical techniques
and less costly than edge notching. A code system must be
that are discussed in Section 7 tend to destroy the corrosion products and
developed when using drilled holes. Punched holes should not
thereby lose the information contained in these corrosion products. Care
be used as they introduce residual strain.
may be required so that uncorroded metal is not removed with the
6.2.5 When it is undesirable to deform the surface of
corrosion products.
specimens after preparation procedures, for example, when
7.1.2 The procedure given in 7.1.1 may not be reliable when
testing coated surfaces, tags may be used for specimen identi-
heavily corroded specimens are to be cleaned. The application
fication. A metal or plastic wire can be used to attach the tag to
of replicate cleaning procedures to specimens with corroded
the specimen and the specimen identification can be stamped
surfaces will often, even in the absence of corrosion products,
on the tag. It is important to ensure that neither the tag nor the
result in continuing mass losses. This is because a corroded
wire will corrode or degrade in the test environment. It is also
surface, particularly of a multiphase alloy, is often more
important to be sure that there are no galvanic interactions
susceptible than a freshly machined or polished surface to
between the tag, wire, and specimen.
corrosion by the cleaning procedure. In such cases, the
6.3 For more searching tests of either the metal or the
following method of determining the mass loss due to the
environment, standard surface finishes may be preferred. A
cleaning procedure is preferred.
suitable procedure might be:
7.1.2.1 The cleaning procedure should be repeated on speci-
6.3.1 Degrease in an organic solvent or hot alkaline cleaner.
mens several times. The mass loss should be determined after
(See also Practice G 31.)
each cleaning by weighing the specimen.
NOTE 2—Hot alkalies and chlorinated solvents may attack some metals.
7.1.2.2 The mass loss should be graphed as a function of the
NOTE 3—Ultrasonic cleaning may be beneficial in both pre-test and
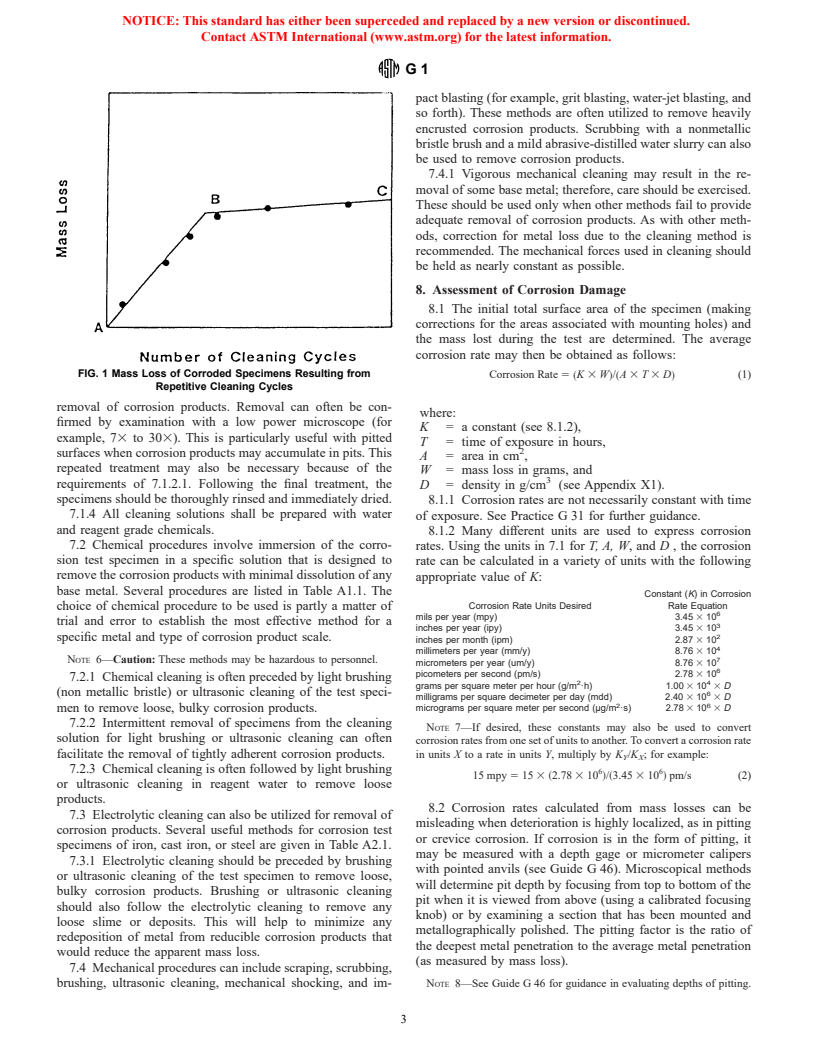
number of equal cleaning cycles as shown in Fig. 1. Two lines
post-test cleaning procedures.
will be obtained: AB and BC. The latter will correspond to
corrosion of the metal after removal of corrosion products. The
6.3.2 Pickle in an appropriate solution if oxides or tarnish
mass loss due to corrosion will correspond approximately to
are present. In some cases the chemical cleaners described in
point B.
Section 6 will suffice.
7.1.2.3 To minimize uncertainty associated with corrosion
NOTE 4—Pickling may cause localized corrosion on some materials.
of the metal by the cleaning method, a method should be
6.3.3 Abrade with a slurry of an appropriate abrasive or with chosen to provide the lowest slope (near to horizontal) of line
an abrasive paper (see Practices A 262 and Test Method BC.
D 1384). The edges as well as the faces of the specimens 7.1.3 Repeated treatment may be required for complete
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
G1
pact blasting (for example, grit blasting, water-jet blasting, and
so forth). These methods are often utilized to remove heavily
encrusted corrosion products. Scrubbing with a nonmetallic
bristle brush and a mild abrasive-distilled water slurry can also
be used to remove corrosion products.
7.4.1 Vigorous mechanical cleaning may result in the re-
moval of some base metal; therefore, care should be exercised.
These should be used only when other methods fail to provide
adequate removal of corrosion products. As with other meth-
ods, correction for metal loss due to the cleaning method is
recommended. The mechanical forces used in cleaning should
be held as nearly constant as possible.
8. Assessment of Corrosion Damage
8.1 The initial total surface area of the specimen (making
corrections for the areas associated with mounting holes) and
the mass lost during the test are determined. The average
corrosion rate may then be obtained as follows:
FIG. 1 Mass Loss of Corroded Specimens Resulting from Corrosion Rate 5 ~K 3 W!/~A 3 T 3 D! (1)
Repetitive Cleaning Cycles
removal of corrosion products. Removal can often be con-
where:
firmed by examination with a low power microscope (for
K = a constant (see 8.1.2),
example, 73 to 303). This is particularly useful with pitted
T = time of exposure in hours,
surfaces when corrosion products may accumulate in pits. This
A = area in cm ,
repeated treatment may also be necessary because of the
W = mass loss in grams, and
requirements of 7.1.2.1. Following the final treatment, the
D = density in g/cm (see Appendix X1).
specimens should be thoroughly rinsed and immediately dried.
8.1.1 Corrosion rates are not necessarily constant with time
7.1.4 All cleaning solutions shall be prepared with water
of exposure. See Practice G 31 for further guidance.
and reagent grade chemicals.
8.1.2 Many different units are used to express corrosion
7.2 Chemical procedures involve immersion of the corro-
rates. Using the units in 7.1 for T, A, W, and D , the corrosion
sion test specimen in a specific solution that is designed to
rate can be calculated in a variety of units with the following
remove the corrosion products with minimal dissolution of any
appropriate value of K:
base metal. Several procedures are listed in Table A1.1. The
Constant (K) in Corrosion
Corrosion Rate Units Desired Rate Equation
choice of chemical procedure to be used is partly a matter of
mils per year (mpy) 3.45 3 10
trial and error to establish the most effective method for a
inches per year (ipy) 3.45 3 10
specific metal and type of corrosion product scale.
inches per month (ipm) 2.87 3 10
millimeters per year (mm/y) 8.76 3 10
NOTE 6—Caution: These methods may be hazardous to personnel. 7
micrometers per year (um/y) 8.76 3 10
picometers per second (pm/s) 2.78 3 10
7.2.1 Chemical cleaning is often preceded by light brushing
2 4
grams per square meter per hour (g/m ·h) 1.00 3 10 3 D
(non metallic bristle) or ultrasonic cleaning of the test speci- 6
milligrams per square decimeter per day (mdd) 2.40 3 10 3 D
2 6
men to remove loose, bulky corrosion products. micrograms per square meter per second (μg/m ·s) 2.78 3 10 3 D
7.2.2 Intermittent removal of specimens from the cleaning
NOTE 7—If desired, these constants may also be used to convert
solution for light brushing or ultrasonic cleaning can often
corrosion rates from one set of units to another. To convert a corrosion rate
facilitate the removal of t
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.