ASTM F2475-05
(Guide)Standard Guide for Biocompatibility Evaluation of Medical Device Packaging Materials
Standard Guide for Biocompatibility Evaluation of Medical Device Packaging Materials
SIGNIFICANCE AND USE
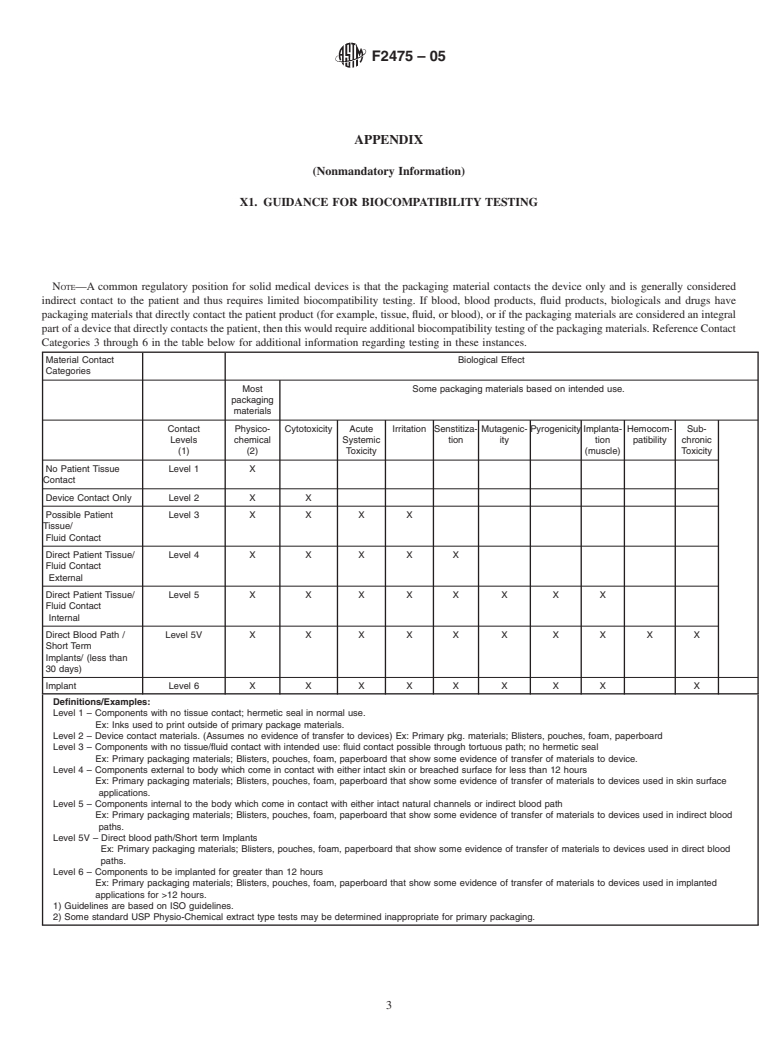
The compatibility of packaging materials with a medical device is a requirement of many regulatory bodies. Since most medical devices are used or implanted in, around or on the human body, these devices must do no harm. Therefore, the packaging materials that come in contact with the medical device must also be evaluated and determined to be safe for use with the human body in that they have no negative impact on the physical, chemical or biological properties of the device. . This evaluation may include both a study of relevant experience with and actual testing of packaging materials. Such an evaluation may result in the conclusion that no testing is needed if the material has a demonstrable history of safe use in the specific role that is the same as that of the package under design.
The medical device manufacturer determines the need for appropriate testing, with consideration of the device/package interactions, if any. The responsibility of the packaging supplier is typically limited to the performance of cytotoxicity testing.
SCOPE
1.1 This guide provides information to determine the appropriate testing for biocompatibility of packaging materials used to contain a medical device.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and to determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2475 – 05
Standard Guide for
Biocompatibility Evaluation of Medical Device Packaging
Materials
This standard is issued under the fixed designation F2475; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.2.1 biocompatibility—the inherent ability of a material to
remain biologically inert with the host in its intended applica-
1.1 This guide provides information to determine the appro-
tion.
priate testing for biocompatibility of packaging materials used
3.2.2 biocompatibility testing—the series of chemical and
to contain a medical device.
biological tests that a material is subjected to in order to
1.2 This standard does not purport to address all of the
determine the ability of the material to remain biologically
safety concerns, if any, associated with its use. It is the
inert with the host in its intended application.
responsibility of the user of this standard to establish appro-
3.2.3 extent of contact—the degree to which the packaged
priate safety and health practices and to determine the
device will contact the patient (refer to ISO 10993-1 for levels
applicability of regulatory limitations prior to use.
of contact of the device with the human body). When referring
2. Referenced Documents
tothepackaging,extentofcontactreferstothedegreetowhich
the packaging will interact with the device. Degree of packag-
2.1 ASTM Standards:
ing contact (interaction) is related to the physical-chemical
F1327 Terminology Relating to Barrier Materials for Medi-
nature of the packaging materials and the device, the intended
cal Packaging
use of the device (levels of contact with the body), and the
2.2 Other Standards:
extent to which the packaging may negatively impact the
ANSI/AAMI/ISO 11607 Packaging for Terminally Steril-
contained device.
ized Medical Devices
ISO 10993-1:2003 Biological Evaluation of Medical De-
4. Summary of Practice
vices – Part 1: Evaluation and Testing
4.1 Materials used in packaging are to be evaluated per
USP <1031> The Biocompatibility of Materials Used in
defined guidelines, such as AAMI/ANSI/ISO 11607. Addi-
Drug Containers, Medical Devices, and Implants
tional biocompatibility testing for packaging materials may be
FDA – Center for Devices and Radiological Health: Re-
required based on the extent of material contact with the
quired Biocompatability Training and Toxicology Profiles
contained medical device, the subsequent degree to which the
for Evaluation of Medical Devices (#G95-1)
packaged device (product) will contact the patient, and the
3. Terminology intendeduseofthedevice.Whenselectingtheappropriatetests
for biological evaluation of medical devices, the chemical
3.1 Definitions—Forterminologyrelatedtobarriermaterials
characteristics of the device materials, as well as the nature,
for medical packaging see Terminology F1327.
degree, frequency and duration of the device’s exposure to the
3.2 Definitions of Terms Specific to This Standard:
body must be considered. Similar testing may be considered
for medical packaging, when there is not a history of safe use
of packaging materials for their intended sue or there may be
This guide is under the jurisdiction of ASTM Committee F02 on Flexible
Barrier Materials and is the direct responsibility of Subcommittee F02.15 on
a question as to whether the packaging may negatively impact
Chemical/Safety Properties.
the contained device. Guidelines for biocompatibility verifica-
Current edition approved April 1, 2005. Published May 2005. DOI: 10.1520/
tion of medical device packaging are based on FDA guidance
F2475-05.
(Memorandum #G-95), ANSI/AAMI/ISO 10993-1 and USP
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
<1031> The Biocompatibility of Materials Used in Drug
Standards volume information, refer to the standard’s Document Summary page on
Containers, Medical Devices, and Implants.While the scope of
the ASTM website.
these standards does not directly apply to medical device
Withdrawn. The last approved version of this historical standard is referenced
on www.astm.org. packaging, use of them will address the intent of ISO 11607.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F2475 – 05
The reader is advised to consult these standards in determin- Variations from standard testing plans may be justified to
ing which tests apply for a given packaging application. All either reduce or expand tests to be done based on 1) known
medicaldevicepackagesareconsideredtohaveindirectpatient potential contact levels of the device with the patient, 2) the
contact, at a minimum. Therefore, the tests selected will not extent of contact between the package and the device, and 3)
typically require more extensive testing than that required for the relative risk that the package may interact with the product,
medical devices intended for indirect patient contact. resulting in a change to the device’s physical, chemical, or
However, test selection should also be based on the extent of biological properties.
contact between the package and the device, and the probabil-
NOTE 1—For semi-solid and liquid device packaging, specific attention
itythatthepackagemaynegativelyimpactthepropertiesofthe
should be paid to the potential for indirect contact components such as
contained medical device. For example, a device that is a solid
inks, varnish and adhesive to volatilize and migrate through the primary
structure is less likely to interact with its packaging than a barrier into the product.
device composed of a semi-solid or liquid material.
The history of use of packages and package materials for
various medical device applications can also serve as a
5. Significance and Use
valuable resource in verifying the biocompatibility of a pack-
5.1 Thecompatibilityofpackagingmaterialswithamedical
age system.
device is a requirement of many regulatory bodies. Since most
6.2 Prepare samples for testing based on testing facilities
medical devices are used or implanted in, around or on the
requirements. Processing steps and labeling of packages can
human body, these devices must do no harm. Therefore, the
impact the biocompatibility of a package system. Therefore, it
packaging materials that come in contact with the medical
is important to test materials that have been manufactured and
devicemustalsobeevaluatedanddeterminedtobesafeforuse
processed under “nominal conditions” as well as worst case
with the human body in that they have no negative impact on
manufacturing conditions, including anti
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.