ASTM E2709-09
(Practice)Standard Practice for Demonstrating Capability to Comply with a Lot Acceptance Procedure
Standard Practice for Demonstrating Capability to Comply with a Lot Acceptance Procedure
SIGNIFICANCE AND USE
Lot acceptance procedures are used in industry for inspecting quality characteristics of raw materials, in-process product, and finished product. These procedures, together with process controls, comprise a quality control program. For additional information on process control see Practice E 2281 dealing with process capability evaluation and Practice E 2587 dealing with the use of control charts in statistical process control.
Lot inspection procedures classify quality characteristics as either attributes (measured on discrete scales such as percent defective) or variables (measured on continuous scales such as length, weight, or concentration).
Operating characteristic curves, which plot the relationship of the lot acceptance probability versus the true lot percent defective, are used to evaluate the discriminatory power of a given lot inspection procedure, or acceptance sampling plan, and are discussed in Practice E 2234.
This practice considers inspection procedures that may involve multiple-stage sampling, where at each stage one can decide to accept the lot or to continue sampling, and the decision to reject the lot is deferred until the last stage.
At each stage there are one or more acceptance criteria on the test results; for example, limits on each individual test result, or limits on statistics based on the sample of test results, such as the average, standard deviation, or coefficient of variation (relative standard deviation).
The methodology in this practice defines an acceptance region for a set of test results from the lot such that, at a prescribed confidence level, the probability that a sample from the lot will pass the original lot acceptance procedure is greater than or equal to a prespecified lower bound.
Having test results fall in the acceptance region is not equivalent to passing the original lot acceptance procedure, but provides assurance that a sample would pass the lot acceptance procedure with a specified probability.
...
SCOPE
1.1 This practice provides a general methodology for evaluating single-stage or multiple-stage lot acceptance procedures which involve a quality characteristic measured on a numerical scale. This methodology computes, at a prescribed confidence level, a lower bound on the probability of passing a lot acceptance procedure, using estimates of the parameters of the distribution of test results from the lot.
1.2 For a prescribed lower probability bound, the methodology can also generate an acceptance limit table, which defines a set of test method outcomes (e.g., sample averages and standard deviations) that would pass the multiple-stage procedure at a prescribed confidence level.
1.3 This approach may be used for demonstrating compliance with in-process, validation, or lot-release specifications.
1.4 The system of units for this practice is not specified.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2709 – 09
Standard Practice for
Demonstrating Capability to Comply with a Lot Acceptance
Procedure
This standard is issued under the fixed designation E2709; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E2587 Practice for Use of Control Charts in Statistical
Process Control
1.1 Thispracticeprovidesageneralmethodologyforevalu-
ating single-stage or multiple-stage lot acceptance procedures
3. Terminology
whichinvolveaqualitycharacteristicmeasuredonanumerical
3.1 Definitions—See Terminology E456 for a more exten-
scale. This methodology computes, at a prescribed confidence
sive listing of terms in ASTM Committee E11 standards.
level, a lower bound on the probability of passing a lot
3.1.1 characteristic, n—a property of items in a sample or
acceptance procedure, using estimates of the parameters of the
population which, when measured, counted or otherwise ob-
distribution of test results from the lot.
served, helps to distinguish between the items. E2282
1.2 For a prescribed lower probability bound, the method-
3.1.2 mean, n— of a population, µ, average or expected
ology can also generate an acceptance limit table, which
valueofacharacteristicinapopulation, of a sample X,sumof
defines a set of test method outcomes (for example, sample
the observed values in a sample divided by the sample size.
averagesandstandarddeviations)thatwouldpassthemultiple-
E2586
stage procedure at a prescribed confidence level.
3.1.3 standard deviation, n—of a population, s, the square
1.3 This approach may be used for demonstrating compli-
root of the average or expected value of the squared deviation
ance with in-process, validation, or lot-release specifications.
of a variable from its mean – of a sample, s, the square root of
1.4 The system of units for this practice is not specified.
thesumofthesquareddeviationsoftheobservedvaluesinthe
1.5 This standard does not purport to address all of the
sample divided by the sample size minus 1. E2586
safety concerns, if any, associated with its use. It is the
3.1.4 test method, n—a definitive procedure that produces a
responsibility of the user of this standard to establish appro-
test result. E2282
priate safety and health practices and determine the applica-
3.2 Definitions of Terms Specific to This Standard:
bility of regulatory limitations prior to use.
3.2.1 acceptable parameter region, n—the set of values of
2. Referenced Documents parameters characterizing the distribution of test results for
2 which the probability of passing the lot acceptance procedure
2.1 ASTM Standards:
is greater than a prescribed lower bound.
E456 Terminology Relating to Quality and Statistics
3.2.2 acceptance region, n—the set of values of parameter
E2234 Practice for Sampling a Stream of Product by
estimates that will attain a prescribed lower bound on the
Attributes Indexed by AQL
probability of passing a lot acceptance procedure at a pre-
E2281 Practice for Process and Measurement Capability
scribed level of confidence.
Indices
3.2.3 acceptance limit, n—the boundary of the acceptance
E2282 Guide for Defining theTest Result of aTest Method
region, for example, the maximum sample standard deviation
E2586 Practice for Calculating and Using Basic Statistics
test results for a given sample mean.
3.2.4 multiple-stage lot acceptance procedure, n—a proce-
dure for accepting a lot that involves more than one stage of
ThispracticeisunderthejurisdictionofASTMCommitteeE11onQualityand
sampling and testing a given quality characteristic and one or
Statistics and is the direct responsibility of Subcommittee E11.30 on Statistical
more acceptance criteria per stage.
Quality Control.
Current edition approved Aug. 1, 2009. Published September 2009. DOI:
4. Significance and Use
10.1520/E2709-09.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
4.1 Lot acceptance procedures are used in industry for
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
inspecting quality characteristics of raw materials, in-process
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. product, and finished product.These procedures, together with
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E2709 – 09
process controls, comprise a quality control program. For 5.1.1 Animportantclassofproceduresisforthecasewhere
additional information on process control see Practice E2281 the quality characteristic is normally distributed. Particular
dealing with process capability evaluation and Practice E2587 instructions for that case are given in this section.
dealing with the use of control charts in statistical process 5.2 Express the probability of passing the given lot accep-
control. tance procedure as a function of parameters characterizing the
4.1.1 Lot inspection procedures classify quality characteris- distribution of the quality characteristic for items in the lot.
tics as either attributes (measured on discrete scales such as 5.2.1 When the characteristic is normally distributed, pa-
percent defective) or variables (measured on continuous scales rametersarethemean(µ)andstandarddeviation(s)ofthelot.
such as length, weight, or concentration). 5.2.2 An expression for the exact probability of passing the
4.1.2 Operating characteristic curves, which plot the rela- lotacceptanceproceduremaybeintractable.Alowerboundfor
tionship of the lot acceptance probability versus the true lot the probability may be used. For multiple stage tests, the
percent defective, are used to evaluate the discriminatory following lower bounds on the probability of passing the
power of a given lot inspection procedure, or acceptance procedure as a function of probabilities of passing stages, and
sampling plan, and are discussed in Practice E2234. ontheprobabilityofpassingastagehavingmultiplecriteriaas
4.2 This practice considers inspection procedures that may a function of the probabilities of passing the criteria, may be
involve multiple-stage sampling, where at each stage one can useful (4).
decide to accept the lot or to continue sampling, and the
P ~pass k– stage procedure!$max $P~S !, P~S !,.,P~S !% (1)
1 2 k
decision to reject the lot is deferred until the last stage.
4.2.1 Ateachstagethereareoneormoreacceptancecriteria where:
P(S) = is the probability of passing stage i, evaluated
on the test results; for example, limits on each individual test
i
regardless of whether previous stages pass or not.
result,orlimitsonstatisticsbasedonthesampleoftestresults,
such as the average, standard deviation, or coefficient of
m
P S ! 5 P C and C .and C !$1– 1– P C !! (2)
~ ~ ~ ~
i i1 i2 im ( j51 ij
variation (relative standard deviation).
4.3 The methodology in this practice defines an acceptance where:
P(C ) = is the probability of passing the j-th criterion of m
region for a set of test results from the lot such that, at a
ij
prescribed confidence level, the probability that a sample from within the i-th stage.
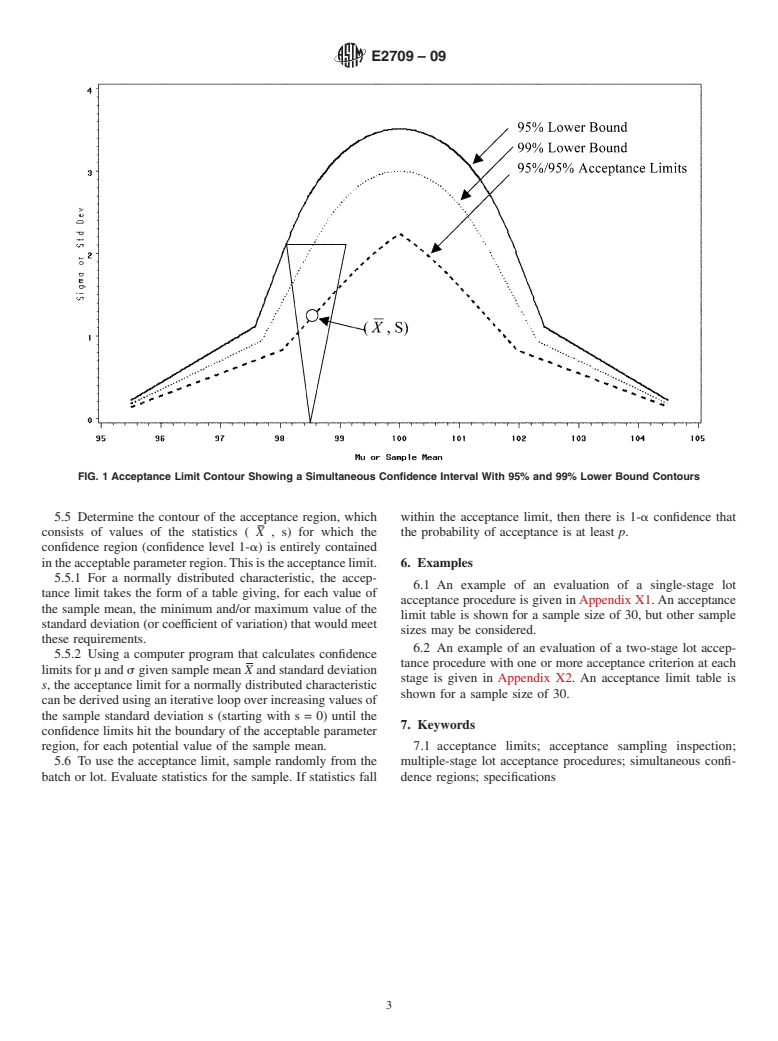
5.3 Determinethecontouroftheregionofparametervalues
thelotwillpasstheoriginallotacceptanceprocedureisgreater
than or equal to a prespecified lower bound. for which the expression for the probability of passing the
given lot acceptance procedure is at least equal to the required
4.3.1 Having test results fall in the acceptance region is not
equivalenttopassingtheoriginallotacceptanceprocedure,but lower bound (LB) on the probability of acceptance (p). This
defines the region of acceptable parameters.
providesassurancethatasamplewouldpassthelotacceptance
procedure with a specified probability. 5.3.1 For a normally distributed population, this will be a
region under a curve in the half-plane where µ is on the
4.3.2 This information can be used for process demonstra-
tion or validation. horizontal axis, s on the vertical axis, such as that depicted in
4.3.3 This information can be used for lot release (accep- Fig. 1.
tance),butthelowerboundmaybeconservativeinsomecases. 5.4 For each value of a statistic or set of statistics, derive a
4.3.4 If the results are to be applied to test results from joint confidence region (confidence coefficient 1-a) for the
future lots from the same process, then it is assumed that the distribution parameters. The size of sample to be taken, n, and
process is in a state of statistical control (see 4.1). If this is not the statistics to be used, must be predetermined.
5.4.1 Foranormallydistributedlot,themethodofLindgren
the case then there can be no guarantee that the probability
estimates would be valid predictions of future process perfor- (6) constructs a simultaneous confidence region of (µ, s)
values from the sample average X and the sample standard
mance.
4.4 This methodology was originally developed by J. S. deviation s from a set of n test results. Let Z and x denote
p p
percentiles of the standard normal distribution and of the
Bergum (1-4) for use in two specific quality characteristics of
drug products in the pharmaceutical industry: content unifor- chi-square distribution with n-1 degrees of freedom, respec-
tively. Given a confidence level 100(1-a), choose d and e such
mityanddissolution,asrespectivelydefinedinchapters<905>
and <711> of the United States Pharmacopeia (5). that (1-a) = (1-2d )(1-e). The values
4.5 Mathematical derivations would be required that are
e51–=1– a
specific to the individual criteria of each test.
and
5. Methodology
d5 ~1– 1– a!/2
=
5.1 The process for defining the acceptance limits, starting
meet this condition. Then
from the definition of the original lot acceptance procedure, is
2 2
X– µ ns
2 2
outlined.Acomputer program is normally required to produce
P #Z P #x 5 ~1–2d!~1–e! 5 ~1– a!
HS D 1– dJ H 2 1– eJ
s/ n
= s
the acceptable parameter region and acceptance limits
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.