ASTM D5156-95
(Test Method)Standard Test Methods for Continuous Measurement of Ozone in Ambient, Workplace, and Indoor Atmospheres (Ultraviolet Absorption)
Standard Test Methods for Continuous Measurement of Ozone in Ambient, Workplace, and Indoor Atmospheres (Ultraviolet Absorption)
SCOPE
1.1 This test method describes the sampling and continuous analysis of ozone (O3) in the atmosphere at concentrations ranging from 10 to 2000 [mu]g/m3 of O3 in air (5 ppb(v) to 1 ppm(v)).
1.1.2 The test method is limited to applications by its sensitivity to interferences as described in Section 6. The interference sensitivities may limit its use for ambient and workplace atmospheres.
1.2 The values stated in SI units are to be regarded as the standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 5156 – 95
Standard Test Methods for
Continuous Measurement of Ozone in Ambient, Workplace,
and Indoor Atmospheres (Ultraviolet Absorption)
This standard is issued under the fixed designation D 5156; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope (SI) (the Modernized Metric System)
E 591 Practice for Safety and Health Requirements Relating
1.1 This test method describes the sampling and continuous
to Occupational Exposure to Ozone
analysis of ozone (O ) in the atmosphere at concentrations
2.2 Other Documents:
ranging from 10 to 2000 μg/m of O in air (5 ppb(v) to 1
EPA-600/4-76-005, Quality Assurance Handbook for Air
ppm(v)).
Pollution Measurement Systems, Vol I, “Principles”
1.1.1 The test method is limited to applications by its
EPA-600/4-77-027a, Quality Assurance Handbook for Air
sensitivity to interferences as described in Section 6. The
Pollution Measurement Systems, Vol II, “Ambient Air
interference sensitivities may limit its use for ambient and
Specific Methods”
workplace atmospheres.
1.2 The values stated in SI units are to be regarded as the
3. Terminology
standard.
3.1 Definitions—For definitions of terms used in this test
1.3 This standard does not purport to address all of the
method, refer to Terminology D 1356. An explanation of units,
safety concerns, if any, associated with its use. It is the
symbols, and conversion factors may be found in Practice
responsibility of the user of this standard to establish appro-
E 380.
priate safety and health practices and determine the applica-
3.2 Definitions of Terms Specific to This Standard:
bility of regulatory limitations prior to use.
3.2.1 absolute ultraviolet photometer—a photometer whose
2. Referenced Documents design, construction, and maintenance is such that it can
measure the absorbance caused by O mixtures without refer-
2.1 ASTM Standards:
ence to external absorption standards. Given a value for the
D 1356 Terminology Relating to Sampling and Analysis of
absorption coefficient of O at 253.7 nm and a reading from the
Atmospheres
absolute ultraviolet photometer, O concentrations can be
D 1357 Practice for Planning the Sampling of the Ambient
calculated with accuracy. An absolute ultraviolet photometer is
Atmosphere
used only on prepared O mixtures free from interferences, as
D 1914 Practice for Conversion Units and Factors Relating
in calibration activity.
to Sampling and Analysis of Atmospheres
3.2.2 primary standard—a standard directly defined and
D 3249 Practice for General Ambient Air Analyzer Proce-
established by some authority, against which all secondary
dures
standards are compared.
D 3631 Test Methods for Measuring Surface Atmospheric
2 3.2.3 secondary standard—a standard used as a means of
Pressure
comparison, but checked against a primary standard.
D 3670 Guide for Determination of Precision and Bias of
2 3.2.4 standard—an accepted reference sample or device
Methods of Committee D-22
used for establishing the measurement of a physical quantity.
D 5011 Practices for Calibration of Ozone Monitors Using
2 3.2.5 transfer standard—a type of secondary standard; it is
Transfer Standards
a transportable device or apparatus that, together with opera-
D 5110 Practice for Calibration of Ozone Monitors and
tional procedures, is capable of reproducing pollutant concen-
Certification of Ozone Transfer Standards Using Ultravio-
trations or producing acceptable assays of pollutant concentra-
let Photometry
tions.
E 380 Practice for Use of the International System of Units
4. Summary of Test Method
These test methods are under the jurisdiction of ASTM Committee D-22 on
4.1 This test method is based on the absorption of ultraviolet
Sampling and Analysis of Atmospheres and is the direct responsibility of Subcom-
mittee D22.03 on Ambient Atmospheres and Source Emissions.
Current edition approved Sept. 10, 1995. Published November 1995. Originally Annual Book of ASTM Standards, Vol 14.02.
published as D 5156 – 91. Last previous edition D 5156 – 91. Discontinued; see 1990 Annual Book of ASTM Standards, Vol 11.03.
2 5
Annual Book of ASTM Standards, Vol 11.03. Available from National Technical Information Service, Springfield, VA 22161.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 5156
radiation at 253.7-nm wavelength by O and the use of an such as lachrymation and burning eyes. Ozone is much more
ozone-specific scrubber to generate a reference air stream with easily monitored than these photochemical oxidants and pro-
only O scrubbed from it. A single-cell ultraviolet absorption vides a good indication of their concentrations, and it is
photometer is used, with the cell filled alternately with ambient therefore the substance that is specified in air quality standards
and O -scrubbed ambient air. The absorption to be measured at and regulations.
the lower part of the operating range is extremely small.
6. Interferences
Special precautions and designs must be used to obtain
accurate results.
6.1 Any aerosol or gas that absorbs or scatters ultraviolet
4.2 The absorption of radiation at 253.7 nm by O at very
radiation at 253.7 nm, and that is removed by the O -specific
low concentrations follows the Beer-Lambert Law. Namely, for
scrubber, constitutes an interferent to this test method (7).
a cell of length d, assuming a constant input ultraviolet
Particulate matter can be removed with a poly-
intensity, the ratio of the emerging intensities for the cell filled
tetrafluoroethylene (PTFE) membrane filter. Any type of filter
with sample air, I , and with O -scrubbed air, I , is:
s 3 o
can, however, become contaminated and may then scrub O .It
I is important to check the O -inertness of such devices fre-
s 3
2~cad!
5 e (1)
I quently.
o
6.2 Some reported positively interfering organic species for
where:
a manganese dioxide scrubber are tabulated in Annex A2 of
c = the concentration of O , ppm (v),
this test method. In general, if interferences are suspected, it is
d = the length of the cell, cm, and
preferable to use another test method rather than to try to scrub
a = the absorption coefficient of O per length unit of d and
out the interfering agent, since the instability of O makes the
per concentration unit of c.
testing and proving of additional interferant scrubbers particu-
4.3 When (cad) is << 1, as is the case for O at 253.7 nm in
larly difficult.
the concentration range specified for this test method, the
6.3 Water vapor may constitute either a positive or negative
approximation
interferant in instruments calibrated with dry span gas (8-11).
2x
e ’ ~1 2 x! (2)
6.3.1 Improperly polished absorption cell windows may
can be used to simplify the signal processing electronics, so lead to increased signal noise and apparent ozone increases in
that
instruments subject to rapidly changing humidity, such as at a
coastal site where instruments may be exposed to frequent
I ’ I ~1 2 cad! (3)
s o
shifts between relatively dry terrestrial and moist oceanic air
and thus
parcels (8).
~I 2 I !
o s 6.3.2 A negative water vapor interference, due to humidity
c ’ (4)
I ad
o
dependent changes in ozone scrubbing efficiency, may develop
4.4 At 1 ppm (v), the high end of the recommended range, in manganese dioxide scrubbers exposed to ambient air (9, 11).
This phenomenon is described in 7.2.6.
and a path length of 50 cm, the error resulting from application
of the above approximation is approximately 1 part in 10 000.
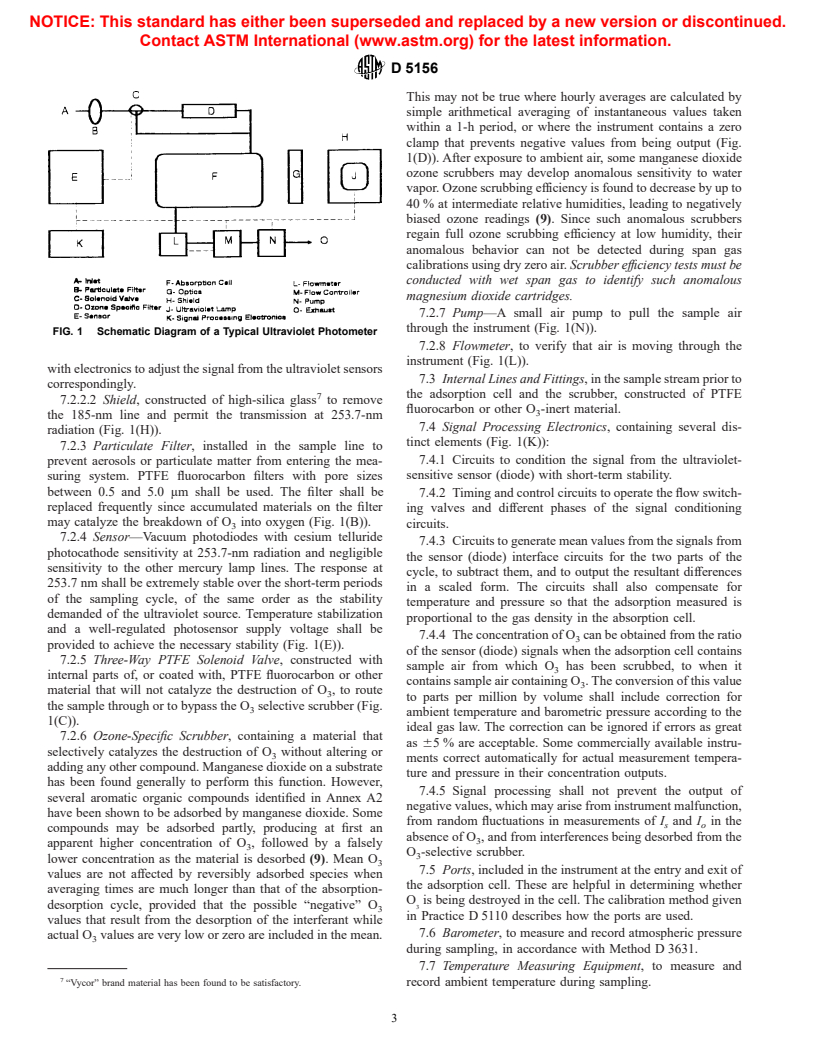
7. Apparatus
4.5 Thus, the concentration of O can be obtained from the
difference between the signal from the photosensor (often a
7.1 Instruments are commercially available that meet the
vacuum photodiode) when the contents of the absorption cell
specifications provided in Annex A1. Note that these specifi-
contain sample air from which O has been scrubbed, and when
cations do not cover operation where the ambient temperature
it contains sample air containing O .
3 changes rapidly.
4.6 At 5 ppb (v) with a 50-cm path length, the absorption is
7.2 The elements of the typical ozone-measuring system are
−6 −5
approximately 308 3 0.005 3 50 3 10 or 3 10 (1-4).
shown in Fig. 1. Assembled, they form a photometric ultravio-
4.7 The instrument is calibrated by methods given in Prac-
let monitor with specifications conforming to those listed in
tices D 5011 and D 5110, which describe the use of an absolute
Annex A1. The components are described in 7.2.1-7.2.8.
ultraviolet photometer as a primary standard and the qualifi-
7.2.1 Ultraviolet Absorption Cell, constructed of materials
cation and use of transfer standards.
inert to O , for example, PTFE-coated metal, borosilicate glass,
and fused silica. It shall be mechanically stable so that the
5. Significance and Use
optical alignments of the source, sensor, and any beam-
5.1 Standards for O in the atmosphere have been promul-
directing devices (mirror, prisms, or lenses) are not affected by
gated by government authorities to protect the health and
changes in ambient temperature (Fig. 1(F)).
welfare of the public (5) and also for the protection of
7.2.2 Ultraviolet Lamp—A low-pressure mercury vapor
industrial workers (6).
discharge lamp enclosed in a shield to prevent its radiation at
5.2 Although O itself is a toxic material, in ambient air it is
185 nm (which generates O ) from reaching the absorption cell
primarily the photochemical oxidants formed along with O in
(Fig. 1(J)).
polluted air exposed to sunlight that cause smog symptoms
7.2.2.1 The lamp output at 253.7 nm shall be extremely
stable, or provision shall be made to compensate for short-term
6 variations at 253.7-nm output, for example, by the use of a
The boldface numbers in parentheses refer to the list of references at the end of
this test method. lamp-intensity monitor to measure output from the lamp and
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 5156
This may not be true where hourly averages are calculated by
simple arithmetical averaging of instantaneous values taken
within a 1-h period, or where the instrument contains a zero
clamp that prevents negative values from being output (Fig.
1(D)). After exposure to ambient air, some manganese dioxide
ozone scrubbers may develop anomalous sensitivity to water
vapor. Ozone scrubbing efficiency is found to decrease by up to
40 % at intermediate relative humidities, leading to negatively
biased ozone readings (9). Since such anomalous scrubbers
regain full ozone scrubbing efficiency at low humidity, their
anomalous behavior can not be detected during span gas
calibrations using dry zero air. Scrubber effıciency tests must be
conducted with wet span gas to identify such anomalous
magnesium dioxide cartridges.
7.2.7 Pump—A small air pump to pull the sample air
through the instrument (Fig. 1(N)).
FIG. 1 Schematic Diagram of a Typical Ultraviolet Photometer
7.2.8 Flowmeter, to verify that air is moving through the
instrument (Fig. 1(L)).
with electronics to adjust the signal from the ultraviolet sensors
7.3 Internal Lines and Fittings, in the sample stream prior to
correspondingly.
the adsorption cell and the scrubber, constructed of PTFE
7.2.2.2 Shield, constructed of high-silica glass to remove
fluorocarbon or other O -inert material.
the 185-nm line and permit the transmission at 253.7-nm
7.4 Signal Processing Electronics, containing several dis-
radiation (Fig. 1(H)).
tinct elements (Fig. 1(K)):
7.2.3 Particulate Filter, installed in the sample line to
prevent aerosols or particulate matter from entering the mea- 7.4.1 Circuits to condition the signal from the ultraviolet-
sensitive sensor (diode) with short-term stability.
suring system. PTFE fluorocarbon filters with pore sizes
between 0.5 and 5.0 μm shall be used. The filter shall be
7.4.2 Timing and control circuits to operate the flow switch-
replaced frequently since accumulated materials on the filter
ing valves and different phases of the signal conditioning
may catalyze the breakdown of O into oxygen (Fig. 1(B)).
circuits.
7.2.4 Sensor—Vacuum photodiodes with cesium telluride
7.4.3 Circuits to generate mean values from the signals from
photocathode sensitivity at 253.7-nm radiation and negligible
the sensor (diode) interface circuits for the two parts of the
sensitivity to the other mercury lamp lines. The response at
cycle, to subtract them, and to output the resultant differences
253.7 nm shall be extremely stable over the short-term periods
in a scaled form. The circuits shall also compensate for
of the sampling cycle, of the same order as the stability
temperature and pressure so that the adsorption measured is
demanded of the ultraviolet source. Temperature stabilization
proportional to the gas density in the absorption cell.
and a well-regulated photosensor supply voltage shall be
7.4.4 The concentration of O can be obtained from the ratio
provided to achieve the necessary stability (Fig. 1(E)).
of the sensor (diode) signals when the adsorption cell contains
7.2.5 Three-Way PTFE Solenoid Valve, constructed with
sample air from which O has been scrubbed, to when it
internal parts of, or coated with, PTFE fluorocarbon or other
contains sample air containing O . The conversion of this value
material that will not catalyze the destruction of O , to route
to parts per million by volume shall include correction for
the sample through or to bypass the O selective scrubber (Fig.
ambient temperature and barometric pressure according to the
1(C)).
ideal gas law. The correction can be ignored if errors as great
7.2.6 Ozone-Specific Scrubber, containing a material that
as 65 % are acceptable. Some commercially available instru-
selectively catalyzes the destruction of O without altering or
ments correct automatically for actual measurement tempera-
adding any other compound. Manganese dioxide on a substrate
ture and pressure in their concentration outputs.
has been found generally to perform this function. However,
7.4.5 Signal processing shall not prevent the output of
several aromatic organic compounds identified in Annex A2
negative values, which may arise from instrument malfunction,
hav
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.