ASTM F2132-01
(Specification)Standard Specification for Puncture Resistance of Materials Used in Containers for Discarded Medical Needles and Other Sharps
Standard Specification for Puncture Resistance of Materials Used in Containers for Discarded Medical Needles and Other Sharps
SCOPE
1.1 The purpose of this specification is to provide a test procedure and performance requirement for the puncture resistance of materials used in the construction of containers for discarded medical needles and other sharps. This test specification will establish (1) the average puncture force and (2) a minimum value of puncture force that container material(s) must withstand when following the test procedure described in Section . This specification shall be applicable to regions of uniform material and thickness, and needle contact areas as defined in and . Materials meeting the performance requirements of Section will be considered "puncture resistant." This specification will not evaluate the construction of, or provide pass/fail criteria for, a sharps container.
1.2 This specification provides a test procedure to determine if all regions of one container meet the material puncture resistance requirements. It does not define the number of additional test containers required to achieve a statistically valid sample of a manufacturing lot or process. An appropriate sampling plan shall be determined by the test requester, as this depends upon the manufacturing process variability, manufacturing lot size, and other factors, such as end-user requirements.
1.3 This specification is intended to evaluate the performance of materials used in the construction or manufacture of sharps containers under controlled laboratory conditions, and at normal room temperature (see ). (Warning—This specification only characterizes material puncture resistance at normal room temperatures. Applications of sharps containers outside the range of 23 2C (beyond the clinical environment, such as usage in emergency vehicles), require further material characterization by the product specifier to determine suitable use.)
1.4 The values stated in inch/pound are to be regarded as the standard. The SI values given in parentheses are for information only.
1.5 The following hazard caveat pertains only to the test procedure portion, Section , of this specification.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 2132 – 01
Standard Specification for
Puncture Resistance of Materials Used in Containers for
Discarded Medical Needles and Other Sharps
This standard is issued under the fixed designation F 2132; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 1.5 The following hazard caveat pertains only to the test
procedure portion, Section 6, of this specification.
1.1 The purpose of this specification is to provide a test
1.6 This standard does not purport to address all of the
procedure and performance requirement for the puncture
safety concerns, if any, associated with its use. It is the
resistance of materials used in the construction of containers
responsibility of the user of this standard to establish appro-
for discarded medical needles and other sharps. This test
priate safety and health practices and determine the applica-
specification will establish (1) the average puncture force and
bility of regulatory limitations prior to use.
(2) a minimum value of puncture force that container materi-
al(s) must withstand when following the test procedure de-
2. Referenced Documents
scribed in Section 6. This specification shall be applicable to
2.1 ASTM Standards:
regions of uniform material and thickness, and needle contact
E 691 Practice for Conducting an Interlaboratory Study to
areas as defined in 3.1.7 and 3.1.9. Materials meeting the
Determine the Precision of a Test Method
performance requirements of Section 4 will be considered
2.2 ISO Standards:
“puncture resistant.” This specification will not evaluate the
ISO 7864 Sterile Hypodermic Needles for Single Use
construction of, or provide pass/fail criteria for, a sharps
ISO 594 Luer Fittings
container.
2.3 Other Standards:
1.2 Thisspecificationprovidesatestproceduretodetermine
AS 4031:1992 Non-reusable Containers for the Collection
if all regions of one container meet the material puncture
of Sharp Medical Items Used in Health Care Areas
resistance requirements. It does not define the number of
BSI 7320:1990 Specification for Sharps Containers
additional test containers required to achieve a statistically
CSA Z316.6-95 Evaluation of Single Use Medical Sharps
valid sample of a manufacturing lot or process.An appropriate
Containers for Biohazardous and Cytotoxic Waste
sampling plan shall be determined by the test requester, as this
DHHS (NIOSH) Publication No. 97-111 Selecting, Evalu-
depends upon the manufacturing process variability, manufac-
ating, and Using Sharps Disposal Containers
turing lot size, and other factors, such as end-user require-
ments.
3. Terminology
1.3 This specification is intended to evaluate the perfor-
3.1 Definitions:
mance of materials used in the construction or manufacture of
3.1.1 container—a product used for the containment of
sharps containers under controlled laboratory conditions, and
discarded medical needles and other sharps.
at normal room temperature (see 6.1). (Warning—This speci-
3.1.2 material—the substance(s) used in the construction of
fication only characterizes material puncture resistance at
a sharps container.
normal room temperatures. Applications of sharps containers
3.1.3 puncture force—the minimum force applied to the
outside the range of 23 6 2°C (beyond the clinical environ-
representative sharp object that causes its tip to penetrate (exit)
ment, such as usage in emergency vehicles), require further
material characterization by the product specifier to determine
suitable use.) 2
Annual Book of ASTM Standards, Vol 14.02.
1.4 Thevaluesstatedininch/poundaretoberegardedasthe
Available from American National Standards Institute, 25 W. 43rd St., 4th
Floor, New York, NY 10036.
standard. The SI values given in parentheses are for informa-
Available from StandardsAustralia International Ltd., 286 Sussex St., Sydney,
tion only.
Australia NSW 2000.
Available from British Standards Institute, 2 Park St., London, England
W1A2B5.
1 6
This specification is under the jurisdiction of ASTM Committee F04 on Available from Canadian Standards Association, Andre Wisaksana, 178
Medical and Surgical Materials and Devices and is the direct responsibility of Rexdale Blvd., Etobicoke, ON Canada M9W 1R3.
Subcommittee F04.33 on Medical/Surgical Instruments. Available from Publications Dissemination, EID National Institute for Occu-
Current edition approved Aug. 10, 2001. Published September 2001. pational Safety and Health, 4676 Columbus Pkwy., Cincinnati, OH 45226-1998.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F 2132
the opposite side of the test specimen from the side that it 5. Sampling and Specimen Preparation
entered when tested in accordance with the test procedure
5.1 Direct Versus Indirect Method—Either of two testing
portion, Section 6, of this specification.
procedures may be used to demonstrate that the material is
3.1.4 puncture resistant—a region of uniform material and
punctureresistantunderthisspecification.Thedirectmethodis
thickness is defined as puncture resistant if it meets Section 4
to be used if the material being evaluated has unknown
of this specification when tested in accordance with Section 6 characteristics. The indirect method may be used only if the
of this specification.
material being evaluated has been previously characterized by
a puncture force versus thickness relationship (see 7.2.2).
3.1.5 test specimen—a sample of material being evaluated
5.1.1 Direct Method Specimen Preparation:
for puncture resistance that is taken from the actual container
5.1.1.1 One sharps container shall be selected at random to
(direct method) or a representative example of the material and
represent the material(s) to be tested. If it is not possible to
thickness having the same characteristics as the actual con-
obtain the required number of test specimens from one
tainer (indirect method). Refer to Section 5.
container, additional randomly selected containers shall be
3.1.6 puncture test specimen—a test specimen that has been
sampled until the required number of test specimens is ob-
punctured using the puncture test described in 6.3, and subse-
tained.
quently evaluated using the direct or indirect methods de-
5.1.1.2 Identify each region of uniform material and thick-
scribed in 7.1 and 7.2 of this specification.
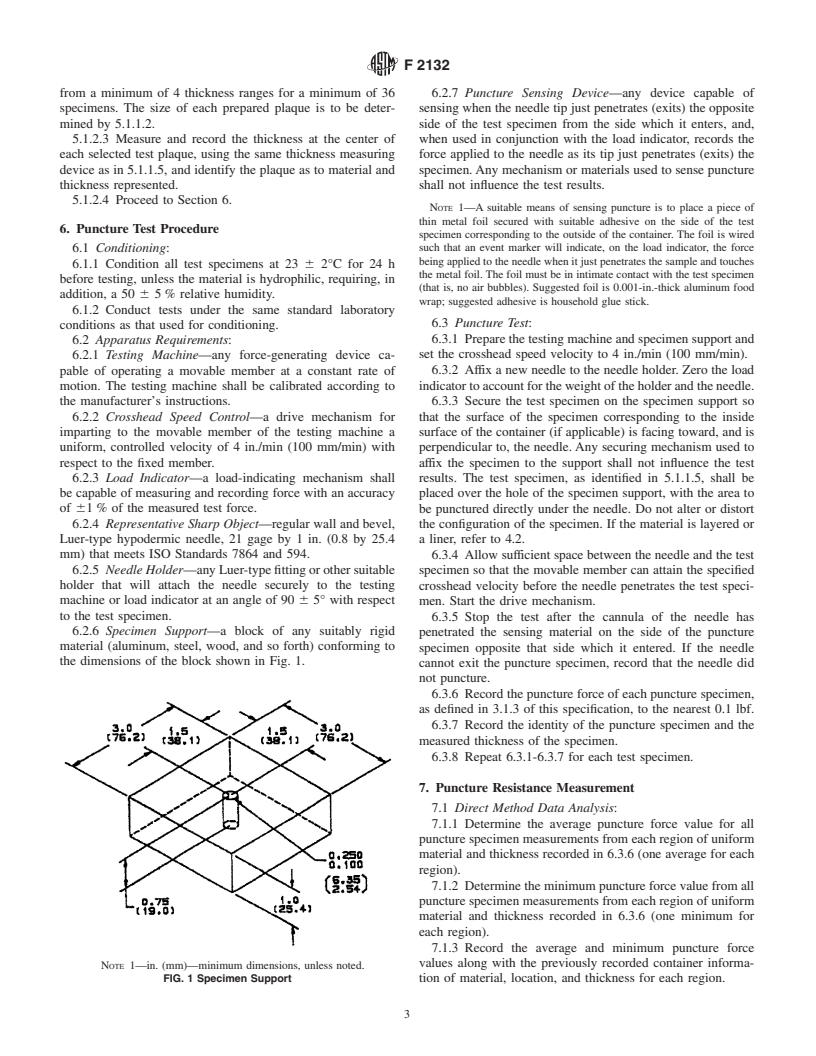
ness(see3.1.7and3.1.9).Markeachregionwithagridof1-in.
3.1.7 region of uniform material and thickness—sharps-
(25.4-mm) squares until the entire region has been covered. If
contactareasofthecontainer,inaggregate,thataremadeofthe
it is not possible to fit a 1-in. grid over certain areas of the
same homogeneous, composite, or laminated material, and, as
container, a smaller grid may be used; however, it shall be no
a consequence of fabrication or design or both, are expected to
less than 0.5 in. (12.7 mm) on a side.
have the same material and thickness as compared to other
5.1.1.3 Number every square of the grid so that each region
areas of the container. For example, in molded containers, the
of uniform material and thickness has consecutive numbers,
corners could be expected to be of different thickness than the
starting with No. 1 in each region.
sides and bottom, resulting in different regions of uniform
5.1.1.4 Using a random-number generator or table, select a
material and thickness. Labels, tabs, membranes, or thin films
quantity of 1-in. (or 0.5-in.) square specimens equal to 10 % of
covering openings in the container are considered separate
the surface area of each region of the container as defined in
regions of uniform material and thickness.
3.1.7 or no less than twelve specimens from each region. If at
3.1.8 sharps—items used in medical treatment, diagnoses,
least twelve specimens cannot be obtained from one container,
or research that may cause puncture wounds, cuts, or tears in
refer to 5.1.1.1. Remove the specimens identified by the
skin or mucous membranes, including, but not limited to:
random number selection from each region of the test con-
hypodermic, surgical, suture, and IV needles; Pasteur pipets,
tainer. Mark the test specimen as it is removed to identify the
lancets, razors, scalpels, and other blades and sharp objects.
inside of the container, as the puncture is required from the
3.1.9 sharps-contact areas—the material of a container that inside of the container outward.
represents those surfaces that enclose sharps within the con- 5.1.1.5 Measure, mark, and record the thickness at the
center of each selected test specimen using a thickness-
tainer, when in its final closure configuration (that is, disposal
configuration). measuring device capable of measuring in 0.001-in. (0.025-
mm) increments, with an accuracy of 2 % of the thickness
measured, for example, a ball micrometer with a ball diameter
4. Performance Requirements
of 0.06 to 0.125 in. (1.6 to 3.2 mm). If the test specimen
4.1 Puncture Resistance Specification—When tested in ac-
includes a radius, corner, edge, or other design feature, find the
cordance with Section 6, the average puncture force to pen-
minimum thickness and mark the location, if not in the center
etrate material test specimens representing any regions of
of the specimen. Identify the specimen as to material and
uniform material and thickness and sharps-contact areas, as
thickness represented.
defined in Section 3, shall not be less than 3.4 lbf (15 N), with
5.1.1.6 Proceed to Section 6.
no one value from any region of material tested less than 2.8
5.1.2 Indirect Method Specimen Preparation:
lbf (12.5 N).
5.1.2.1 Obtainfabricatedormoldedtestspecimens(referred
4.2 Layered Materials and Liners—If a container is de-
to as plaques within the indirect section) representing each
signed to use nonlaminated layers of material in sharps-contact
material, range of thickness, and equivalent manufacturing
areas, the combination of these layered materials must be
process used to represent the sharps container. These plaques
tested as configured in actual use, and meet the puncture
will not be from the container itself, but will be used to
resistance specification of this standard to be deemed puncture
correlate the measured thickness of an actual container to the
resistant. If a container is designed to use a removable liner
puncture resistance value of plaques having the same charac-
enclosed by the container, the material used in the removable
teristics.
liner must meet the puncture resistance specification of this
5.1.2.2 Produce a minimum of nine test plaques to represent
standard to be deemed puncture resistant.
a minimum of four different thicknesses that span the range of
For example, layered materials must be tested with the same
thicknesses expected for each region of uniform material and
spacing as configured in the actual application. thickness of the representative container. Select 9 test plaques
F 2132
from a minimum of 4 thickness ranges for a minimum of 36 6.2.7 Puncture Sensing Device—any device capable of
specimens. The size of each prepared plaque is to be deter- sensing when the needle tip just penetrates (exits) the opposite
mined by 5.1.1.2. side of the test specimen from the side which it enters, and,
5.1.2.3 Measure and record the thickness at the center of when used in conjunction with the load indicator, records the
each selected test plaque, using the same thickness measuring force applied to the needle as its tip just penetrates (exits) the
device as in 5.1.1.5, and identify the plaque as to material and specimen.Any mechanism or materials used to sense puncture
thickness represented. shall not influence the test results.
5.1.2.4 Proceed to Section 6.
NOTE 1—A suitable means of sensing puncture is to place a piece of
thin metal foil secured with suitable adhesive on the side of the test
6. Puncture Test Procedure
specimen corresponding to the outside of the container. The foil is wired
such that an event marker will indicate, on the load indicator, the force
6.1 Conditioning:
being applied to the needle when it just penetrates the sample and touches
6.1.1 Condition all test specimens at 23 6 2°C for 24 h
the metal foil. The foil must be in intimate contact with the test specimen
before testing, unless the material is hydrophilic, requiring, in
(that is, no air bubbles). Suggested foil is 0.001-in.-thick aluminum food
addition, a 50 6 5 % relative humidity.
wrap; suggested adhesive is household glue stick.
6.1.2 Conduct tests under the same standard laboratory
6.3 Puncture Test:
conditions as that used for conditioning.
6.3.1 Prepare the testing machine and specimen support and
6.2 Apparatus Requirements:
set the crosshead speed velocity to 4 in./min (100 mm/min).
6.2.1 Testing Machine—any force-generating device ca-
6.3.2 Affix a new needle to the needle holder. Zero the load
pable of operating a movable member at a constant rate of
motion. The testing machine shall be calibrated according to indicatortoaccountfortheweightoftheholderandtheneedle.
the manufacturer’s instructions. 6.3.3 Secure the test specimen on the specimen support so
6.2.2 Crosshead Speed Control—a drive mechanism for that the surface of the specimen corresponding to the inside
imparting to the movable member of the testing machine a
surface of the container (if applicable) is facing toward, and is
uniform, controlled velocity of 4 in./min (100 mm/min) with perpendicular to, the needle. Any securing mechanism used to
respect to the fixed member.
affix the specimen to the support shall not influence the test
6.2.3 Load Indicator—a load-indicating mechanism shall results. The test specimen, as identified in 5.1.1.5, shall be
be capable of measuring and recording force with an accuracy placed over the hole of the specimen support, with the area to
of 61 % of the measured test force. be punctured directly under the needle. Do not alter or distort
6.2.4 Representative Sharp Object—regular wall and bevel, the configuration of the specimen. If the material is layered or
Luer-type hypodermic needle, 21 gage by 1 in. (0.8 by 25.4 a liner, refer to 4.2.
mm) that meets ISO Standards 7864 and 594.
6.3.4 Allow sufficient space between the needle and the test
6.2.5 Needle Holder—anyLuer-typefittingorothersuitable specimen so that the movable member can attain the specified
holder that will attach the needle securely to the testing
crosshead velocity before the needle penetrates the test speci-
machine or load indicator at an angle of 90 6 5° with respect men. Start the drive mechanism.
to
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.